- Services Overview
- Analytes Details
- FAQ
What is Human Liver Toxicity?
Human liver toxicity encompasses a spectrum of adverse effects that substances can inflict on liver cells, disrupting their normal functions and potentially leading to significant health issues. The liver, being the body's primary metabolic organ, plays a crucial role in detoxifying various compounds, metabolizing drugs, and regulating biochemical processes essential for maintaining homeostasis.
When toxic substances—whether pharmaceuticals, environmental pollutants, or dietary components—enter the body, the liver is often the first line of defense. Unfortunately, this vital organ is susceptible to damage due to its central role in metabolizing these compounds. Hepatotoxicity, a specific form of liver toxicity, can manifest in several ways, including:
- Cellular Injury: Toxic agents can cause direct damage to hepatocytes (liver cells), leading to cell death and inflammation. This damage can compromise liver function and promote the development of conditions such as steatosis (fatty liver) and fibrosis.
- Cholestasis: Some substances can disrupt bile flow, leading to cholestasis, which results in the accumulation of bile acids in the liver and bloodstream. This condition can further exacerbate liver injury and affect overall health.
- Immune Response Activation: Exposure to certain toxins may trigger an immune response, leading to autoimmune hepatitis, where the body's immune system mistakenly attacks liver cells, causing inflammation and damage.
- Liver Failure: In severe cases, extensive liver damage can progress to liver failure, a life-threatening condition requiring urgent medical intervention, including potential liver transplantation.
The implications of human liver toxicity extend beyond individual health concerns. It poses significant challenges in drug development and public health. Regulatory bodies require comprehensive toxicity testing to ensure the safety of new drugs, emphasizing the need for early detection and evaluation of liver toxicity.
Recent research highlights the importance of personalized medicine and genetic predisposition in assessing liver toxicity. Factors such as age, sex, genetic polymorphisms, and pre-existing liver conditions can influence an individual's susceptibility to liver damage. Understanding the mechanisms and biomarkers associated with liver toxicity is vital for developing effective therapeutic strategies and improving patient safety.
Human Liver Toxicity Panel at Creative Proteomics
Creative Proteomics provides advanced Human Liver Toxicity Panels that deliver in-depth analysis of liver metabolism and potential hepatotoxicity. Our 5-plex panel offers a targeted approach to assess liver enzyme activity, making it ideal for identifying early indicators of liver toxicity.
For a more comprehensive evaluation, we also offer a 6-plex panel that enhances the analysis by incorporating additional targets. This expanded panel allows researchers to gain deeper insights into the effects of various substances on liver health. Both panels utilize Luminex xMAP technology, ensuring high sensitivity and specificity in the results.
Detection Method
Magnetic bead-based Luminex multiplex assay
Species
Human
Analytes Detected
| Species | Specification | Protein Targets | Applications | Price |
|---|---|---|---|---|
| Human | Human Liver Toxicity 5-plex Panel | CYP2D6, CYP2C19, CYP2E1, B2M, GAPDH | Suitable for assessing liver enzyme activity and identifying potential hepatotoxicity. | +Inquiry |
| Human | Human Liver Toxicity 6-plex Panel | CYP1A2, CYP3A4, CYP2B6, CYP2C9, B2M, GAPDH | Ideal for comprehensive evaluation of liver metabolism and toxicity profiles. | +Inquiry |
Advantages of the Human Liver Toxicity Luminex Assay
- Multiplexing Capability: The assay can detect up to 6 biomarkers simultaneously from a single sample, significantly reducing sample volume requirements by approximately 80% compared to single-analyte assays.
- High Sensitivity and Specificity: The assay boasts a sensitivity of <1 pg/mL for many liver toxicity biomarkers, enabling the detection of low-level changes that might indicate early liver damage.
- Rapid Turnaround Time: Results are typically available within 24 to 48 hours, allowing researchers to make informed decisions more quickly than traditional methods, which can take several days or even weeks.
- Comprehensive Data: By providing a detailed profile of liver toxicity, the assay can analyze multiple pathways and mechanisms, facilitating a more holistic understanding of substance effects.
- Cost-Effectiveness: Researchers can save up to 30% in costs when utilizing the multiplexing capabilities of the Luminex assay compared to running individual tests for each biomarker.
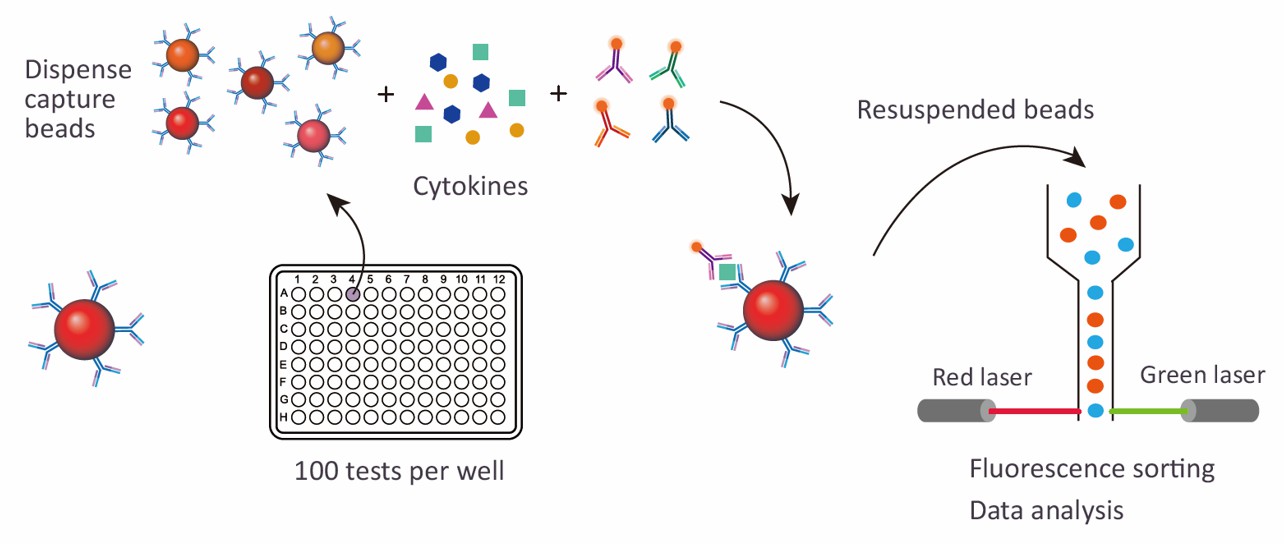
Sample Requirements for Human Liver Toxicity Luminex Panel
| Sample Type | Volume Required | Storage Conditions |
|---|---|---|
| Serum | 100 µL | -80°C for long-term storage |
| Plasma | 100 µL | -80°C for long-term storage |
| Liver Tissue | 50 mg | -80°C for long-term storage |
| Cell Culture Supernatant | 200 µL | -80°C for long-term storage |
| Whole Blood | 200 µL | -80°C for long-term storage |
| Urine | 500 µL | -20°C for long-term storage |
| Saliva | 500 µL | -80°C for long-term storage |
| Bile | 100 µL | -80°C for long-term storage |
Application of Human Liver Toxicity Panel
- Pharmaceutical Research and Development
In drug development, assessing liver toxicity is a critical step to ensure that new drugs are safe for further testing and eventual human use. The Human Liver Toxicity Panel aids in:
- Identifying Potential Hepatotoxic Compounds: Early-stage screening of drug candidates for liver toxicity helps in identifying and discarding potentially harmful compounds, thereby saving time and resources.
- Mechanistic Studies: Understanding the mechanism of liver injury induced by different chemical compounds aids in designing safer drugs and in post-marketing surveillance.
- Regulatory Compliance: Meeting guidelines and requirements set by regulatory bodies such as the FDA and EMA, which mandate rigorous testing for liver toxicity.
- Biomedical Research
In academic and clinical research settings, the panel is used to understand liver disease mechanisms and to develop new therapies:
- Pathophysiological Studies: Helps researchers investigate the underlying mechanisms of liver diseases, including hepatitis, cirrhosis, and liver cancer.
- Biomarker Discovery: Identification and validation of new biomarkers for early diagnosis, prognosis, and monitoring of liver diseases.
- Environmental Toxicology
Environmental toxicologists use the Human Liver Toxicity Panel to assess the impact of environmental contaminants on liver health:
- Toxicological Screening: Evaluation of chemicals, pesticides, and other environmental pollutants for hepatotoxicity.
- Ecotoxicology Studies: Investigation of the effects of environmental toxins on wildlife and ecosystems, which indirectly can affect human health.
- Food and Nutraceutical Safety
The food and nutraceutical industries utilize the Human Liver Toxicity Panel to ensure the safety of their products:
- Safety Assessment: Screening food additives, supplements, and other ingredients for potential liver toxicity.
- Quality Control: Continuous monitoring and testing of product batches to ensure they do not compromise liver function upon consumption.
In addition to preconfigured panels, we also offer customized analysis services. You can customize your own panel through our customization tool, or directly email us the targets you are interested in. A professional will contact you to discuss the feasibility of customization. We look forward to working with you!
| Protein Target | Description |
|---|---|
| CYP2D6 | A key enzyme in drug metabolism, CYP2D6 is crucial for the bioactivation and clearance of a wide range of pharmaceuticals, influencing therapeutic efficacy and safety. |
| CYP2C19 | This enzyme is vital for the metabolic processing of several critical drugs, including antiplatelets and proton pump inhibitors, affecting their therapeutic outcomes. |
| CYP2E1 | Known for its role in metabolizing ethanol and various xenobiotics, CYP2E1 is linked to oxidative stress and liver injury, highlighting its significance in toxicity assessments. |
| B2M | As a sensitive marker of tissue damage and inflammation, B2M provides insights into renal function and immune response, making it valuable in evaluating liver health. |
| GAPDH | Serving as a reliable housekeeping gene, GAPDH is essential for data normalization in assays, ensuring the accuracy and consistency of experimental results. |
| CYP1A2 | This enzyme plays a crucial role in detoxifying environmental pollutants and metabolizing various medications, making it essential for understanding exposure risks. |
| CYP3A4 | The most prevalent cytochrome P450 enzyme, CYP3A4 is responsible for metabolizing approximately 50% of all drugs, impacting both drug interactions and patient responses. |
| CYP2B6 | Involved in the metabolism of numerous drugs, CYP2B6's activity is critical for understanding drug efficacy and potential toxicity, especially in diverse patient populations. |
| CYP2C9 | This enzyme is significant for metabolizing nonsteroidal anti-inflammatory drugs (NSAIDs) and anticoagulants, influencing their therapeutic effects and safety profiles. |
| B2M | As a biomarker of tissue integrity, B2M indicates inflammatory processes and kidney function, providing a broader context for assessing liver-related health. |
| GAPDH | Essential for ensuring consistent assay results, GAPDH serves as a standard reference for normalization, reinforcing the reliability of the data obtained. |
How can I determine which panel is best suited for my research needs?
Choosing the appropriate panel depends on your specific research objectives. The 5-plex panel is ideal for initial assessments and early-stage screening of drug candidates for liver toxicity. It focuses on key enzymes involved in drug metabolism. If your research requires a more comprehensive understanding of liver metabolism and toxicity profiles, the 6-plex panel is recommended, as it includes additional biomarkers that provide deeper insights into hepatic function and potential toxic effects. Consider factors such as the complexity of your compounds and the specific endpoints you wish to investigate when making your selection.
What is the significance of using Luminex xMAP technology in these assays?
Luminex xMAP technology enables the simultaneous detection of multiple analytes from a single sample, which significantly enhances the efficiency and sensitivity of the analysis. This technology utilizes color-coded beads coated with specific capture antibodies, allowing for precise identification and quantification of biomarkers. The high sensitivity (<1 pg/mL) means that even subtle changes in liver function can be detected early, which is crucial for timely intervention and decision-making in both research and clinical settings.
How are results from the Luminex assay validated?
Results from the Human Liver Toxicity Panels are validated through multiple quality control measures. These include running standard curves and controls in each assay batch, ensuring the consistency and reliability of data. Additionally, we employ internal validations, comparing results with established methods and literature benchmarks to confirm accuracy. This rigorous approach enhances confidence in the findings, which is crucial for both research and regulatory compliance.

