Sample types
Commonly serum, plasma, and cell-culture supernatant; other matrices can be evaluated as needed.
More insights. One well.
Magnetic, bead-based multiplex assay kits for multi-analyte quantification across key pathways and species—built to accelerate confident decisions.
Get a Quote
Run more markers with less sample. Our Magnetic Multiplex Assay Kits quantify multiple targets in a single well using xMAP-compatible, bead-based immunoassay technology—ideal for discovery, pathway mapping, and translational research.
1. Choose Species
Human, Mouse, Rat, Non-human Primate, Chicken (Gallus), etc.
2. Choose Category
3. Choose N-plex
Select the multiplex size that balances coverage and throughput. You can compare kits or request a custom configuration.
Plan low-µL sample inputs and appropriate dilutions based on expected concentrations and plex size. A same-day incubate–wash–detect–read sequence is typical. Use blanks and QC controls; include a standard curve spanning the working range.
1. Capture — Add sample to a color-coded magnetic bead mix; each bead population carries a capture antibody for one target.
2. Detect — Add biotinylated detection antibodies to form sandwiches; add reporter to generate quantitative fluorescence.
3. Readout — Acquire on an xMAP-compatible instrument. The system resolves bead regions (which target) and reporter intensity (how much), then fits a logistic curve to report concentrations.


Sample types
Commonly serum, plasma, and cell-culture supernatant; other matrices can be evaluated as needed.

Sample input
Low-volume setup with defined dilution practices to protect limited material.

Assay time
Streamlined magnetic protocol suitable for medium-to-high throughput.

Dynamic range & sensitivity
Broad working ranges with pg/mL-level sensitivity depending on target and matrix.

Precision
Intra-/inter-assay precision monitored across plates and lots; acceptance windows defined before release.

Readout
Compatible with standard xMAP instruments; routine acquisition settings apply.

How do I decide between different plex sizes?
Start from your required analytes, then select the smallest plex size that includes them. Larger plexes expand coverage but may require more careful optimization of dilutions to ensure all targets fall within the quantifiable range.
Can I customize the analyte panel?
Yes. If the stocked kits do not fully match your research needs, you can request a custom configuration. Common approaches include adding or removing certain analytes, or tailoring a panel to a specific pathway or disease area.
Which sample matrices are recommended?
Serum, plasma, and cell culture supernatant are the most commonly validated matrices. For other sample types (e.g., CSF, BAL fluid, tissue lysates), we recommend pilot tests with dilution series and spike-and-recovery checks to confirm performance.
How much sample volume is required per well?
The assay is designed for low-volume inputs, typically in the µL range per well. Actual volume depends on plex size and instrument settings, but most studies can be run with limited material, making the kits suitable for precious clinical or preclinical samples.
What controls should I include in my run?
Always include blanks, a full standard curve, and at least one quality-control sample or calibrator across plates. This ensures consistent quantification and helps monitor assay drift over time.
How reproducible are results across different lots?
Each lot is released only after meeting predefined criteria for standard curve performance, precision, and bead coupling. Certificates of Analysis (CoA) are available where applicable to document lot performance.
Can I automate the workflow?
Yes. The magnetic bead format is compatible with 96-well magnets and most liquid-handling systems. Many labs integrate these kits into semi-automated or fully automated immunoassay workflows.
What is the expected lifetime of reconstituted reagents?
Once reconstituted, reagents should be used promptly and stored according to handling instructions. Stability after reconstitution is limited, so avoid repeated freeze–thaw cycles to maintain performance.
Are results comparable across species panels?
Panels are species-specific, but results can often be compared in relative terms across species. However, absolute values may differ due to antibody affinities and calibrator standards. For true cross-species comparisons, plan parallel runs with harmonized assay conditions.
How should I analyze data from high-plex panels?
Use curve-fitting software capable of 4PL or 5PL logistic regression with appropriate weighting. Apply QC rules (CV limits, recovery ranges) consistently, and avoid extrapolating beyond the reportable range.








Luminex instruments leverage xMAP multiplexing to sensitively and efficiently measure hundreds of analytes in a single run.
Read More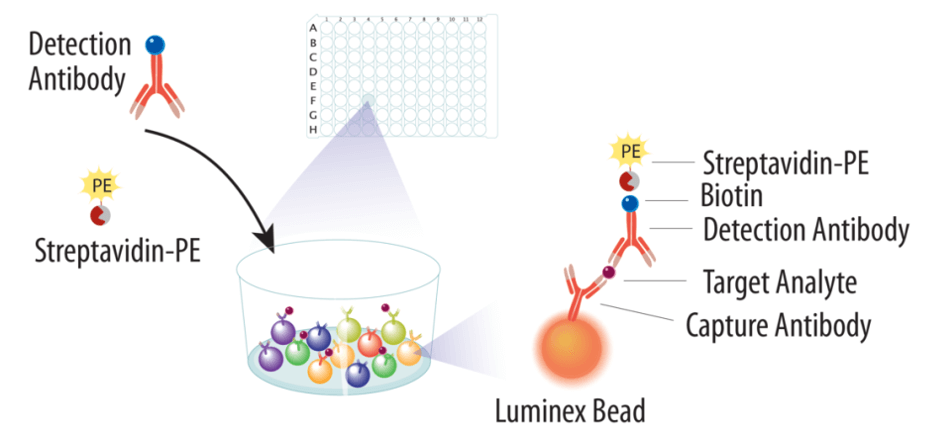
This page offers essential guidelines for collecting samples for accurate Luminex multiplex analysis.
Read More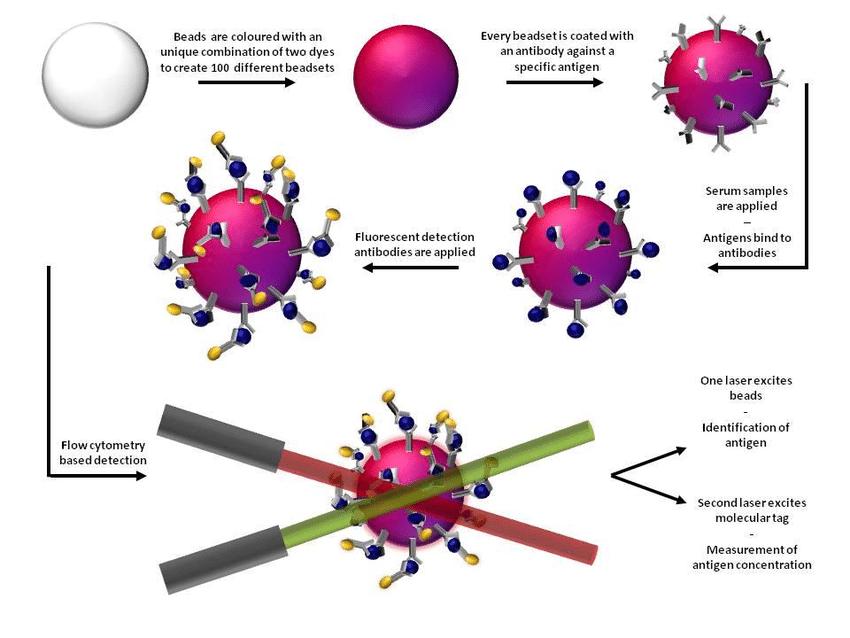
Explore the differences between Luminex xMAP and xTAG technologies, crucial for understanding their applications in biomolecule analysis.
Read MoreOnline Inquiry
×