Introduction to autophagy signaling pathway
Based on Luminex technology platform, Creative Proteomics provides analysis services for key targets of autophagy signaling pathway.
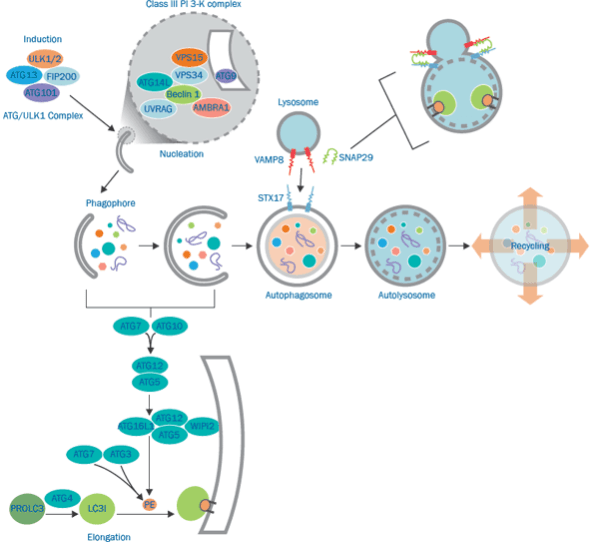 Autophagy-related proteins (ATG) regulate this process in yeast and many ATG proteins are conserved in mammals. Individual ATG proteins and ATG-complexes support specific steps in the dynamic process of autophagy. The formation of a double membrane vesicle for the entrapment and delivery of cytosolic content to lysosomes is the hallmark of autophagy. Formation of the mature organelle or autolysosome involves a sequence of coordinated events. In each step, ATG proteins catalyze specific reactions critical for the maintenance of autophagic flux.
Autophagy-related proteins (ATG) regulate this process in yeast and many ATG proteins are conserved in mammals. Individual ATG proteins and ATG-complexes support specific steps in the dynamic process of autophagy. The formation of a double membrane vesicle for the entrapment and delivery of cytosolic content to lysosomes is the hallmark of autophagy. Formation of the mature organelle or autolysosome involves a sequence of coordinated events. In each step, ATG proteins catalyze specific reactions critical for the maintenance of autophagic flux.
Autophagy has recently been shown to be an important component of the innate immune response. The signaling pathways leading to activation of autophagy in innate immunity are not well studied.Recent study shows that Toll-like receptor 4 (TLR 4) serves as an environmental sensor for autophagy. Defined a new molecular pathway in which lipopolysaccharide (LPS) induces autophagy in human and murine macrophages by a pathway regulated through Toll-interleukin 1 receptor domain-containing adaptor-inducing interferon-β (TRIF)-dependent, myeloid differentiation factor 88 (MyD88)-independent TLR4 signaling. Receptor-interacting protein (RIP1) and p38 mitogen-activated protein-kinase (MAPK) are downstream components of this pathway. This signaling pathway does not affect cell viability, indicating that it is distinct from an autophagic death signaling pathway. Further show that LPS-induced autophagy can enhance mycobacterial co-localization with the autophagosomes.
Regulatory pathways of autophagy signaling pathway:
- Autophagy that relies on the mTOR (mammalian target of rapamycin) pathway
- PI3K-AKT-mTOR signal pathway
- AMPK-TSC1/2-mTOR signal pathway
- Other signal pathways
- 3-Methyladenine (3-MA) inhibits autophagy by inhibiting the activity of Class Ⅲ PI3K.
- Beclin1 and UVRAG act as positive regulators, and the anti-apoptotic factor bcl-2 as negative regulators participate in the formation of Class Ⅲ PI3 complex to regulate autophagy.
- GTP-bound G protein subunit Gαi3 inhibits autophagy; GDP-bound Gαi3 protein activates autophagy.
- Death-associated protein linase (DAPK) and DAPK-related protein kinase-1 (DRP-1) induce autophagy.
Our detectable targets:
| ATG13 | ATG2 | ATG12 | ATG5 | ATG7 | ATG10 |
| ATG3 | ATG4 | JNK | MYD88 | Rac1 | TLR4 |
| ATG16 | WIPi2 | LC3I | PROLC3 | ULK | FIP200 |
| VPS15 | VPS34 | UYRAG | NFκB | RIG-1 | TRAF3 |
| GAS | IRF9 | AMBRA1 | p38 | RIP1 | TRAF5 |
| Histone-H3 | IRF5 | MEK3 | p38MAPK | SH2 | TRAF6 |
| IKK-α | IRS1 | MEK6 | p50 | SLP76 | TRAM |
| IKK-β | IRS2 | MSK1 | p65 | Tak1 | TRIF |
| IPS-1 | ISGF3 | MSK2 | PI3K | TBK1 | Vav |
| IRAK1 | ISRE | mTOR | PKR | TLR3 |
Technology platform:
We provide Luminex technology for autophagy signaling pathway analysis.
Luminex technology is a multifunctional liquid phase analysis platform developed on the basis of colored microspheres, laser technology, applied fluidics and high-speed digital signal processing technology. The core is to encode polypropylene microspheres or magnetic microspheres with fluorescent dyes. By adjusting the different ratios of the two fluorescent dyes, up to 100 microspheres with different fluorescence spectra can be obtained. Antigen-antibody, enzyme-substrate, ligand-receptor binding reactions and nucleic acid hybridization reactions are performed on microspheres with different fluorescence encoding. Qualitative and quantitative analysis by laser detection of microsphere coding and reporter fluorescence separately.
The hallmark of autophagy is the formation of double-membrane vesicles, which are used to trap cytoplasmic content in the lysosome and deliver it to the lysosome. Autophagy signaling proteins play a vital role in maintaining autophagy flux, regulating innate immunity, and removing pathogens.
In addition to Luminex Multiplex Assay, Enzyme-linked immunosorbent assay (ELISA), Flow cytometry (FACS analysis) technology can also be provided to meet other customer needs.
Advantages of autophagy signaling pathway detection:
- High sensitivity: The surface area of the microspheres is large, and 100,000 probes can be fixed on each microsphere, which ensures the maximum binding with the target molecules in the sample and improves the detection sensitivity. The lowest detection concentration can reach 0.1pg/mL.
- Strong specificity: Can read the fluorescent signal on a single microsphere, and automatically distinguish between bound and unbound microspheres.
- High accuracy: Without washing, the fluorescence intensity of the reporter molecule on the microsphere is proportional to the bound molecule to be detected. Because the detection range of the Luminex detection platform is large, it does not need to be diluted like ELISA detection, which further reduces errors.
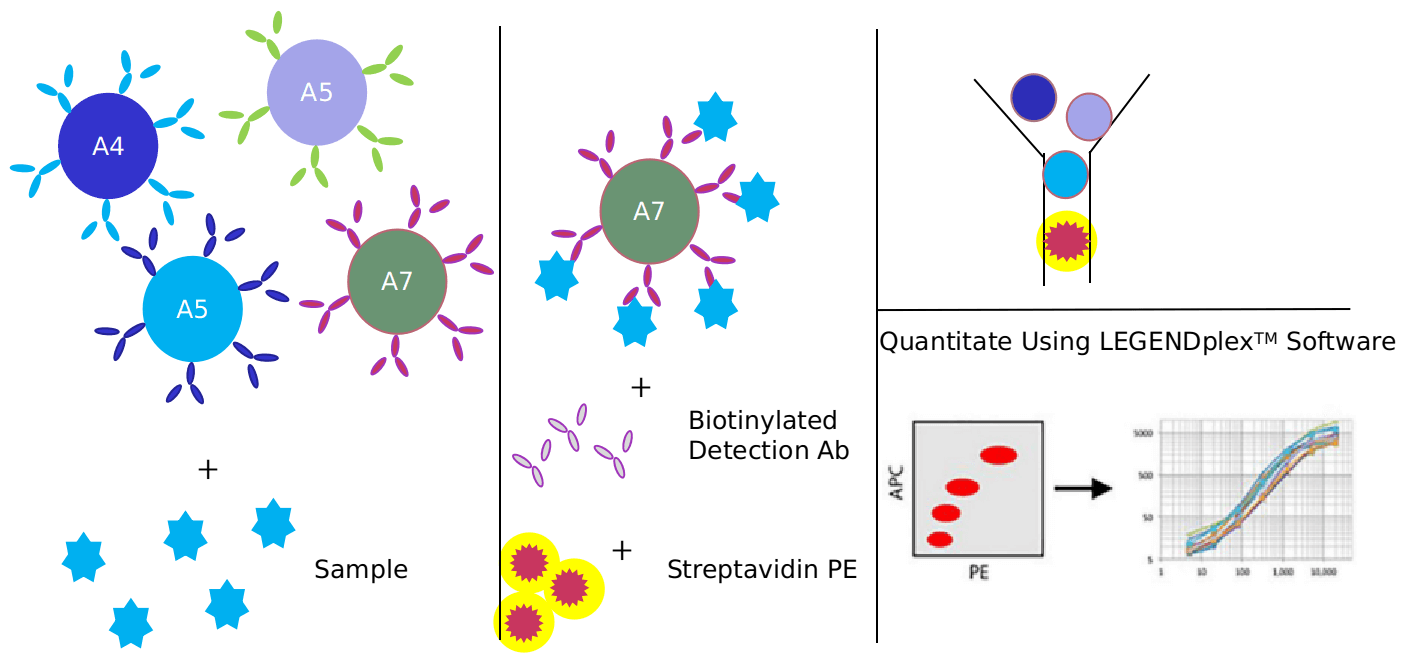
Application of our service:
- To study the regulation mechanism of autophagy signal pathway in disease
- To study the effect of each virus on autophagy signaling pathway
- To study the effects of drugs or therapies on autophagy signaling pathways
Creative Proteomics has developed a signal pathway target detection platform. We are not limited to providing autophagy signal path detection services, but can also provide other signal path detection services. If you want to detect other targets, please contact us and we will customize the service for you. Look forward to working with you.
References:
- Ji-Won Lee, Hyeri Nam, et al. TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia. Autophagy, 2019, 15(5): 753-770.
- Ming Chen, Jiaxing Liu, et al. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy, 2017, 13(11): 1813-1827.
- Romana T. Netea-Maier, et al. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy, 2016, 12(2): 245-260.

