- Service Details
- Case Study
Serum & Plasma Sample for Cytokine Analysis
Cytokines are a class of highly active, multifunctional, soluble, small molecule proteins secreted by activated immune cells and certain stromal cells (e.g., bone marrow stromal cells). Serum and plasma samples are the most common biological samples used for cytokine testing. Serum is a clear, pale yellow liquid separated from the inside of blood (after centrifugation or resting) and does not contain fibrinogen and is suitable for clinical chemistry and clinical immunology tests. Plasma contains blood cells and their organic components as well as a variety of physiological substances and metabolites. Based on the Luminex platform, Creative Proteomics can provide you with cytokine and protein molecule assays for serum or plasma samples.
We can provide you with:
- Ultra-low abundance cytokine and chemokine assays
- Rapid quantification of multiple proteins simultaneously
- Protein biomarker analysis in serum or plasma samples
Why Choose Serum and Plasma for Cytokine Analysis?
Comprehensive Representation of Systemic Responses
Serum and plasma samples provide a holistic view of systemic responses. These liquid components of blood circulate throughout the entire body, offering a comprehensive representation of the intricate cytokine networks at play. This approach ensures that the analysis captures the full spectrum of cellular communication, enabling a more nuanced understanding of physiological processes.
Non-Invasive Sampling for Longitudinal Studies
Serum and plasma sampling is a non-invasive method, making it ideal for longitudinal studies and large-scale population analyses. This approach allows for the collection of samples over time without causing discomfort to individuals, facilitating a deeper exploration of cytokine profiles across diverse cohorts.
Stability Ensuring Reliable Results
Stability is key to maintaining sample integrity. Serum and plasma samples exhibit excellent stability, even during storage and transportation. This characteristic is crucial for ensuring the reliability and reproducibility of cytokine analyses. At Creative Proteomics, we prioritize the preservation of sample integrity, guaranteeing the accuracy of your results.
Real-Time Insights into Physiological States
Dynamic changes in cytokine levels offer real-time insights. The dynamic nature of cytokine levels in serum and plasma enables real-time monitoring of physiological states. This feature is invaluable for tracking disease progression, assessing the efficacy of treatments, and monitoring recovery. Creative Proteomics harnesses this capability to provide analyses that go beyond static data.
Compatibility with High-Throughput Techniques
Seamless integration with high-throughput techniques enhances efficiency. Advancements in technology have enabled high-throughput cytokine analysis. Serum and plasma samples seamlessly integrate with these cutting-edge techniques, allowing for the simultaneous quantification of multiple cytokines with high sensitivity and precision. This ensures efficiency and accuracy in your analysis.
Technology Platform for Cytokine Analysis
We mainly provide the Luminex cytokine detection platform. Luminex uses fluorescently encoded microspheres with specific antibodies to different target molecules. The different microspheres can be combined freely to a certain extent so that up to 100 analytes can be tested multiple times simultaneously in a single experiment.
The Luminex cytokine assay platform has the following advantages:
- Multiple detection: simultaneous detection of 100 biological targets
- Short experiment time: 1-4 weeks
- High sensitivity: the lower limit of accurate quantification is as low as 0.1 pg/mL
- Save samples: only need a sample volume as low as 25 μL
- Time saving: the experiment process only takes 4 hours
For your different needs, we can also provide the following detection methods:
- Simoa
- Meso scale discovery (MSD)
- Enzyme-linked immunosorbent assay (ELISA)
- Flow cytometry
Sample Requirements for Serum/Plasma Cytokine Analysis
| Sample volume | 25 μL of plasma or serum per sample | |
| Sample preparation method | Plasma |
1) Collect 2 mL of peripheral blood and quickly transfer to a heparin anticoagulation tube (green-tipped cap tube); |
| Serum |
1) Collect whole blood into vacuum blood collection tubes (without anticoagulant, red cap tubes), serum tubes should be mixed by gently inverting 180 degrees up and down for 5-6 times; |
|
* Notes:
- Hemolysis should be avoided during the sampling process.
- Heparin is the recommended anticoagulant, EDTA is not recommended. EDTA may have an effect on subsequent mass spectrometry tests.
- Repeated freezing and thawing of blood used for the separation of serum and plasma should be avoided.
Creative Proteomics can provide you with a one-stop solution for cytokine and protein detection and quantification. If you would like to learn more, please contact us. We look forward to working with you.
Case Comparison of Luminex Multiplex Assays with Conventional ELISAs for Biomarker Measurement in Obesity-Related Disorders
Background
Obesity, a growing epidemic in the United States, is associated with various health risks, including metabolic syndrome and cardiovascular diseases. The study aims to evaluate the efficacy of Luminex multiplex assays, a novel technology, in comparison to traditional ELISAs for measuring biomarkers linked to obesity, inflammation, and related disorders.
Samples
The research involved 80 obese individuals enrolled in a weight loss program. Blood samples, collected before and after rapid weight loss, were analyzed for various biomarkers associated with obesity and inflammation. The participants, exhibiting a mean age of 47.1 years and a mean body mass index of 38.3 kg/m2, provided plasma samples that were stored at -70 °C for subsequent analyses.
Technical Methods
The study employed Luminex-100 bead-based systems for multiplexed proteomics research, allowing simultaneous measurement of multiple proteins in a small sample volume (25–50 μL). Various panels, such as the Linco Human Endocrine 3-Plex Panel and Biosource Chemokine 5-Plex Panel, were utilized to measure analytes like leptin, insulin, C-peptide, MCP-1, eotaxin, TNF-α, IL-8, and IL-6. Calibration microspheres with unique color codes anchored with capture antibodies facilitated quantification.
ELISAs were performed using commercially available reagent sets for comparison. Detection limits, recovery studies, and precision analyses (both within-run and between-run) were conducted for both Luminex assays and ELISAs. Statistical analyses were performed using SPSS, Deming regression, Passing–Bablok analyses, and Bland–Altman analyses.
Results
Detection Limits and Sensitivity:
- Luminex assays demonstrated varied detection limits compared to ELISAs.
- Functional sensitivity was emphasized, highlighting challenges in detecting certain cytokines with the multiplex method.
Undetectable Concentrations:
- Percentage of samples with undetectable concentrations varied between Luminex and ELISA methods.
- Notably, some cytokines had high percentages of undetectable concentrations in the Luminex assay.
Precision and Imprecision:
- Within-run imprecision for Luminex assays was generally <15%, but between-run imprecision for MCP-1 exceeded 15%, indicating caution in its use.
- ELISAs exhibited acceptable precision, and imprecision values were within reasonable limits.
Correlation Analyses:
- Correlation coefficients between Luminex assays and ELISAs varied for different analytes.
- Leptin, insulin, MCP-1, and eotaxin showed good correlations, while TNF-α, IL-8, and IL-6 exhibited poor correlations.
Clinical Application and Total Error:
- Despite challenges, both Luminex and ELISA methods provided similar information about relative changes in biomarker concentrations associated with obesity and metabolic syndrome.
- Total error considerations were discussed, emphasizing the clinical research context.
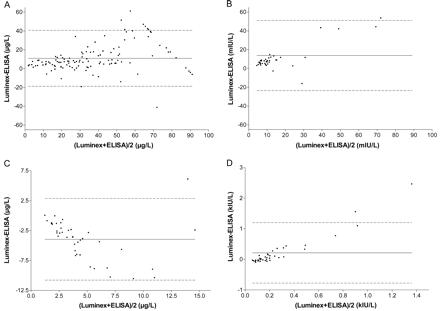 Bland–Altman analysis showing the agreement between Luminex assays and ELISAs for the measurement of leptin (A), insulin (B), C-peptide (C), and MCP-1 (D).
Bland–Altman analysis showing the agreement between Luminex assays and ELISAs for the measurement of leptin (A), insulin (B), C-peptide (C), and MCP-1 (D).
Reference:
- Liu, Mine Y., et al. "Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system." Clinical chemistry 51.7 (2005): 1102-1109.

