Introduction
IL-33 is a multifunctional cytokine discovered in 2005. It was isolated from high-walled endothelial cells. It was found that its protein sequence is similar to the canine DVS27 protein sequence and is located in the nucleus of high-walled endothelial cells. Nuclear factor derived from parietal endothelial cells. Subsequently, the study found that its gene sequence and structure are similar to members of the IL-1 family, IL-1β and IL-18, and are new members of the IL-1 family. IL-33 can be localized in the nucleus to play the role of a transcription factor, or it can transmit activation signals to the cell through ST2 and related signaling protein Acp receptor complexes located on the cell membrane surface, and activate MAPK through a series of signal transmission Inducing the release of effector factors such as IL-5, activating mast cells, lymphocytes, and eosinophils to produce Th2 cytokines, plays a very important role in many diseases such as inflammation and infection.
Mechanism and Function
It was first discovered that IL-33 was a nuclear factor, and it was later found to be a ligand for ST2. On the one hand, as a molecule located in the nucleus, it regulates gene expression and acts as a transcription factor. On the other hand, it is secreted extracellularly, and binds to the surrounding cells or the receptor molecule of the secreting cell itself, playing the role of cytokine. The human IL-33 gene is located on chromosome 9 with a relative molecular mass of about 18,000 and is composed of 270 amino acids. The mouse IL-33 gene is located on chromosome 19 and consists of 266 amino acids. The protein sequences of the two are about 50% similar, but the three-dimensional structure is more similar. IL-33 has a helix-turn-helix structure at the amino terminus, and there is a core sequence that determines the binding of IL-33 to heterochromatin in the nucleus. The carboxy terminus is the binding site of IL-33 to the receptor and has a β-clover structure homologous to the IL-1 cytokine family.
The trans-model ST2L and receptor accessory protein (AcP) together constitute the membrane surface receptor of IL-33. The receptors of IL-1 molecules are composed of two chains, one of which is responsible for binding to the ligand, and the other is responsible for the activation of the signal to the cell. ST2 is responsible for binding to IL-33, while AcP is the signaling chain. IL-33 binds heterodimers composed of ST2 and AcP on the cell membrane to transmit signals into the cell, recruiting downstream signaling molecules myeloid differentiation factor 88 (MyD88), IL-1 receptor related kinase (IRAK) and tumor necrosis Factor receptor-associated factor 6 (TRAF6) also activates downstream mitogen-activated protein kinase (MAPKK), which activates AP-1 through c-junN-terminal kinase (JNK). TRAF6 can also activate the nuclear factor NF-kB kinase inhibitor complex, leading to the release of NF-kB from the complex, and ultimately lead to the production and function of Th2 cytokines IL-4, IL-5 and IL-13.
As a cytokine, IL-33 plays an important role by interacting with its receptor. IL-33 can polarize naive T cells to produce Th2 cytokines such as IL-4, IL-5 and IL-13, and can also play a chemotactic role as a chemokine for Th2 cells. IL-33 can also cause mast cells and basophils to secrete inflammatory cytokines and chemokines, resulting in NK cells and NKT cells secreting Th1-type cytokines. In addition, IL-33 can enhance the differentiation of M2 macrophages, induce the maturation of dendritic cells, and promote the Th1-type response, but it depends on the local cytokine environment.
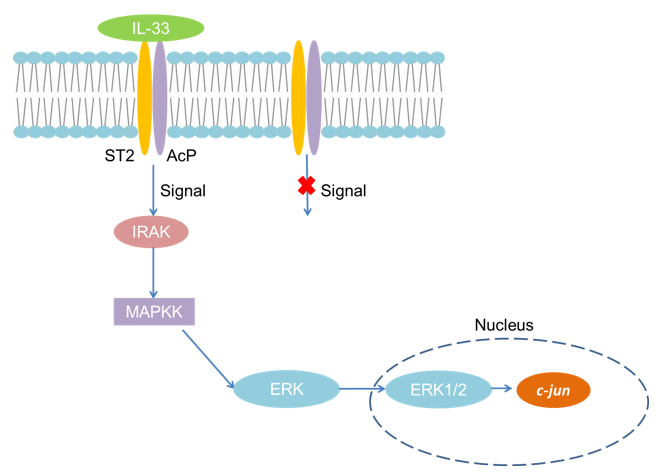 Fig 1. Mechanism of Signaling
Fig 1. Mechanism of Signaling
Creative Proteomics can provide cytokine detection platform for scientific research. According to different purposes, our dedicated analysts will customize exclusive solutions for you. We aim to provide customers with high-quality and convenient services to help you accelerate the progress of your project.
Our cytokine detection service includes but is not limited to:
- One or more cytokines qualitative and quantitative detection
- Cytokines qualitative and quantitative detection of various species
- Cytokine antibodies qualitative and quantitative detection
Sample requirements
- Sample Types-Blood, serum, plasma, cell culture medium, tissue homogenate, etc.
- Sample Volume - It is optimal for 50 samples. This volume allows for triplicate testing of each sample.
Our advantages:
- Different detection methods can be selected based on different samples and requirements.
- Ensure the specificity and accuracy of the test by using high quality antibodies.
- Repeat the test to ensure the repeatability and accuracy of the experimental results.
- Feedback results are accurate and efficient.
Technology platform:
We mainly provide the Luminex cytokine detection platform. Luminex uses fluorescently encoded microspheres with specific antibodies to different target molecules. The different microspheres can be combined freely to a certain extent so that up to 100 analytes can be tested multiple times simultaneously in a single experiment.
The Luminex cytokine assay platform has the following advantages:
- Multiple detection: simultaneous detection of 100 biological targets
- Short experiment time: 1-3 weeks
- High sensitivity: the lower limit of accurate quantification is as low as 0.1 pg/mL
- Save samples: only need a sample volume as low as 25 μL
- Time saving: the experiment process only takes 4 hours
For your different needs, we can also provide the following detection methods:
- Enzyme-linked immunosorbent assay (ELISA)
- Flow cytometry
Workflow

For more information about the IL-33 detection service or need other detection requirements, please contact us.
References:
- Roussel L, et al. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep, 2008, 9(10):1006-1012.
- Luthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity, 2009, 31(1):84-98.

