Introduction
In 1975, Carswell discovered a factor in the serum that causes tumor necrosis in animals called tumor necrosis factor (TNF). Subsequently, a large amount of research has been carried out on the structure, composition and various biological activities of TNF. It is now known that TNF is a cytokine with a variety of biological activities. According to their different sources, they can be divided into two categories: TNF-α (vaginin) produced by macrophages; and TNF-β (lymphytoxin) produced by activated lymphocytes.
Mechanism and Function
As a cytokine, TNF needs to bind to cell surface receptors to complete the transmission of cellular signals. After years of human exploration and research results, human TNF receptors (TNFR) can be divided into two categories: TNFR1 and TNFR2,they are type I transmembrane proteins. TNFR1 is widely distributed on most cell surfaces in vivo, and the expression of TNFR2 is mainly localized on the surface of immune cells. The binding of intracellular domains and death domains of TNFR1 are silenced before TNFR1 binds to TNF. The process inhibits the binding of the death domain (DD) of TNFRI to a linker molecule such as TNFR1, and blocks apoptosis induced by TNFR1 activation. When the tripolymer of TNPα binds to the extracellular domain of TNFR1, causing trimerization of TNFRI, the silencer of death domains(SODD) of the intracellular domain is released. The intracellular death domain of TNFR1 is combined with the binding protein of the TRADD death region, which can initiate three pathway:
- NF-κB pathway: Activated TRADD recruits TRAF2 and RIP. The recruited TRAF2 can further recruit the multi-component protein kinase IKK. The recruited RIP has serine-threonine kinase activity, which can activate IKK. Activated IKK phosphorylates IκBα, which normally binds to NF-κB to inhibit its activity, and phosphorylated IκBα is subsequently degraded and releases NF-κB. The released NF-κB mediates a large number of involved cell survival and proliferation, inflammatory responses and transcription of anti-apoptotic functional proteins.
- MAPK pathway: In the three major cascades of MAPK, TNF induces activation of the stress-related JNK pathway, the response of the p38-MAPK pathway, and the classical ERKs pathway. The recruited TRAF2/Rac activates MLK2/MLK3, TAK1, MEKK1 and ASK1 kinase upstream of the JNK pathway. These kinases phosphorylate MKK7, which in turn activates the JNK pathway. When JNK enters the nucleus, it further activates transcription factors such as c-Jun and ATF2. These transcription factors regulate the expression of genes involved in cell differentiation, proliferation, and apoptosis.
- Induction of death signals: Similar to other family members of the TNFR family that contain the death domain, TNFR1 is also involved in the induction of death signals. However, TNF-induced cell death only accounts for a small fraction of its body function compared to its important role in the inflammatory process in the body. In addition, TNFR1 death-inducing ability is weaker than other family members and is often antagonized by NF-κB. However, when TRADD binds to FADD, FADD recruits the cysteine protease caspase-8. The recruited caspase-8 can be activated by its own proteolytic activity, which in turn activates the caspase-mediated apoptotic pathway leading to apoptosis.
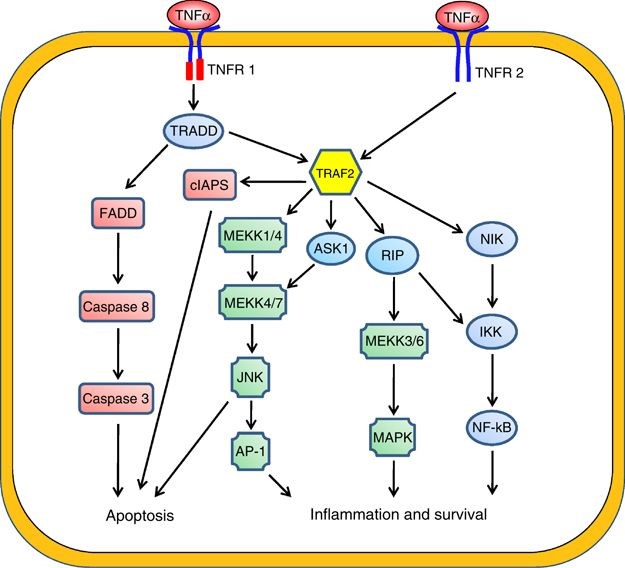 Figure 1. The downstream signalling pathways of TNF-α.
Figure 1. The downstream signalling pathways of TNF-α.
Creative Proteomics can provide cytokine detection platform for scientific research. According to different purposes, our dedicated analysts will customize exclusive solutions for you. We aim to provide customers with high-quality and convenient services to help you accelerate the progress of your project.
Our cytokines include but are not limited to:
- Qualitative and quantitative detection of single cytokines
- Qualitative and quantitative detection of multiple cytokines
- Qualitative and quantitative detection of multiple species of cytokines
- Cytokine antibody qualitative and quantitative detection
Sample requirements
- Sample Types-Blood, serum, plasma, cell culture supernatant, cell lysate, cell culture medium, tissue homogenate, urine, tumor, etc.
- Sample Volume - It is optimal for at least 200 μl per sample. This allows the volume of each sample was tested in triplicate.
Our advantages:
- There are many detection methods, may be selected based on different samples and requirements.
- Detection process using high-quality antibodies, in order to improve the specificity and accuracy of detection.
- Repeat the test to ensure that the results of repeatability and accuracy.
- Professional and efficient feedback results.
Technology platform:
We mainly provide the Luminex cytokine detection platform. Luminex uses fluorescently encoded microspheres with specific antibodies to different target molecules. The different microspheres can be combined freely to a certain extent so that up to 100 analytes can be tested multiple times simultaneously in a single experiment.
The Luminex cytokine assay platform has the following advantages:
- Multiple detection: simultaneous detection of 100 biological targets
- Short experiment time: 1-3 weeks
- High sensitivity: the lower limit of accurate quantification is as low as 0.1 pg/mL
- Save samples: only need a sample volume as low as 25 μL
- Time saving: the experiment process only takes 4 hours
For your different needs, we can also provide the following detection methods:
- Enzyme-linked immunosorbent assay (ELISA)
- Flow cytometry
Workflow
 Figure 2. The workflow of TNF-α 1 detection service.
Figure 2. The workflow of TNF-α 1 detection service.
For more information about the TNF-α detection service or need other detection requirements, please contact us.
References:
- Locksley RM, et al. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001(4): 487–501.
- Pennica D, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984, 312 (5996): 724–9.
- Y Wu& B P Zhou. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. British Journal of Cancer. 2010,102: 639–644.

