- Services Overview
- Analytes Details
- FAQ
What is Human Atherosclerosis?
Human atherosclerosis is a chronic inflammatory disease affecting the cardiovascular system, characterized by the buildup of lipids, inflammatory cells, and fibrous tissue within the arterial walls. This condition begins with endothelial dysfunction, often triggered by risk factors such as high levels of low-density lipoprotein (LDL) cholesterol, hypertension, smoking, diabetes, and obesity. When the endothelium is damaged, it becomes permeable to LDL cholesterol, allowing it to infiltrate the arterial wall.
Once inside the wall, LDL undergoes oxidation, which triggers an inflammatory response. Immune cells, primarily monocytes, are recruited to the site of injury. These monocytes differentiate into macrophages, which engulf the oxidized LDL, transforming into foam cells. As these foam cells accumulate, they form fatty streaks that evolve into larger plaques. The presence of inflammatory cytokines further exacerbates this process, promoting smooth muscle cell proliferation and collagen deposition, which contribute to plaque stability.
As the atherosclerotic plaques grow, they can undergo calcification and become fibrous, leading to a narrowing of the arteries, known as stenosis. This impairs blood flow and can result in ischemia. The plaques can also become unstable and rupture, exposing their contents to the bloodstream. This can lead to the formation of a thrombus (blood clot), which may completely occlude the artery, causing serious cardiovascular events such as myocardial infarction or stroke.
The significance of analyzing atherosclerosis lies in its complexity and prevalence. Understanding the underlying mechanisms can aid in identifying biomarkers that reflect the disease's progression and severity. This knowledge is crucial for developing effective therapeutic strategies. By employing advanced analytical techniques, such as those provided by Creative Proteomics, researchers can gain deeper insights into the biological processes of atherosclerosis, facilitating the discovery of targeted interventions and ultimately improving cardiovascular health outcomes.
Human Atherosclerosis Panel at Creative Proteomics
Creative Proteomics offers a comprehensive Human Atherosclerosis 4-plex/9-plex Panel designed to provide in-depth analysis of key biomarkers associated with atherosclerosis. Utilizing advanced Luminex multiplexing technology, this panel allows for the simultaneous measurement of various protein targets, enabling researchers to gain valuable insights into the disease's mechanisms and progression.
Detection Method
Magnetic bead-based Luminex multiplex assay
Species
Human
Analytes Detected
| Species | Specification | Protein Targets | Applications | Price |
|---|---|---|---|---|
| Human | Human Atherosclerosis 4-plex Panel | Adiponectin, ICAM, Lp-PLA2, YKL-40 | Suitable for analyzing inflammatory markers and metabolic dysfunction related to atherosclerosis. | +Inquiry |
| Human | Human Atherosclerosis 9-plex Panel | FGF-2, HSP60, IL-6, IL-8 (CXCL8), LOX-1, Myeloperoxidase (MPO), PAI-1 (Serpin), Tenascin-C, TRAIL | Ideal for studying atherosclerosis-related inflammation, cellular stress, and plaque instability. | +Inquiry |
Advantages of the Human Atherosclerosis Luminex Assay
- Multiplexing Efficiency: The assay measures multiple biomarkers simultaneously, providing a comprehensive view of atherosclerosis-related processes such as inflammation, lipid metabolism, and endothelial dysfunction in a single run.
- High Sensitivity and Specificity: Luminex technology detects low-abundance proteins with high accuracy, capturing critical biomarker changes even at early stages of atherosclerosis.
- Minimal Sample Volume: Only small sample volumes are required, making it ideal for limited or valuable samples, such as from small animal models or rare patient populations.
- High Throughput: The platform enables the rapid processing of many samples at once, increasing efficiency for large-scale studies and biomarker screenings.
- Cost-Effective: By combining multiple analytes into one assay, the Luminex platform saves on time and reagent costs, making it cost-efficient for extensive research.
- Customizable and Scalable: The assay is flexible and can be tailored to include specific biomarkers, making it suitable for both targeted and broad research applications.
- Reliable and Reproducible: Luminex provides consistent, robust results, ensuring reliability across experiments and labs, making it ideal for long-term studies.
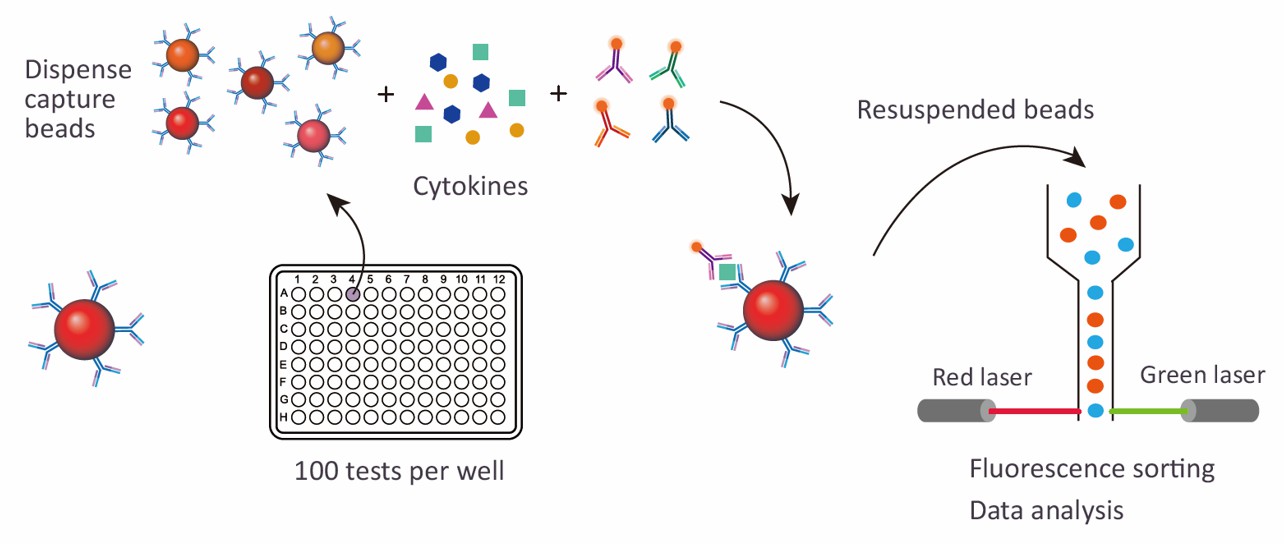
Sample Requirements for Human Atherosclerosis Luminex Panel
| Sample Type | Volume Required | Collection Method | Storage Conditions |
|---|---|---|---|
| Serum | 200 µL | Venipuncture | -80°C for long-term storage |
| Plasma | 200 µL | EDTA or Citrate tubes | -80°C for long-term storage |
| Whole Blood | 500 µL | EDTA or Citrate tubes | Analyze within 24 hours |
| Tissue Samples | 50 mg | Fresh or snap-frozen | -80°C for long-term storage |
| Urine | 10 mL | Clean catch | -20°C for long-term storage |
| Saliva | 1 mL | Passive drool or swab | -20°C for long-term storage |
| Lymphatic Fluid | 200 µL | Collected via lymphatic puncture | -80°C for long-term storage |
| Synovial Fluid | 200 µL | Arthrocentesis | -80°C for long-term storage |
| Cerebrospinal Fluid | 500 µL | Lumbar puncture | -80°C for long-term storage |
| Bronchoalveolar Lavage | 1 mL | Bronchoscopy | -80°C for long-term storage |
Application of Human Atherosclerosis Panel
- Mechanistic Research
The panel facilitates the investigation of biological pathways involved in atherosclerosis, helping to clarify the molecular interactions and inflammatory responses that drive plaque formation and stability.
- Inflammation Studies
By measuring inflammatory markers, researchers can assess the role of chronic inflammation in atherosclerosis, identifying potential targets for anti-inflammatory therapies.
- Biomarker Discovery
The panel supports the identification and validation of novel biomarkers associated with atherosclerosis, enhancing understanding of disease progression and therapeutic responses.
- Environmental Impact Studies
Researchers can analyze how lifestyle factors and environmental exposures affect biomarker levels, shedding light on modifiable risk factors in atherosclerosis development.
- Drug Development
In the drug development process, the panel allows for the evaluation of how new therapeutic agents influence biomarker profiles, providing insights into their mechanisms of action.
- Animal Model Studies
The panel can be applied in preclinical animal models to assess the effects of genetic modifications or treatments on atherosclerosis-related biomarkers, aiding in hypothesis testing.
- Systems Biology
By integrating biomarker data into systems biology frameworks, researchers can model complex interactions in atherosclerosis, enhancing understanding of disease dynamics.
In addition to preconfigured panels, we also offer customized analysis services. You can customize your own panel through our customization tool, or directly email us the targets you are interested in. A professional will contact you to discuss the feasibility of customization. We look forward to working with you!
| Protein Target | Description |
|---|---|
| Adiponectin | An anti-inflammatory adipokine that plays a crucial role in regulating glucose levels and fatty acid breakdown, often linked to reduced cardiovascular risk. |
| ICAM | Intercellular Adhesion Molecule, involved in the inflammatory response and leukocyte adhesion, serves as a marker for endothelial activation in atherosclerosis. |
| Lp-PLA2 | Lipoprotein-associated phospholipase A2, an enzyme that contributes to lipid metabolism and is associated with inflammation and plaque instability in atherosclerosis. |
| YKL-40 | A glycoprotein linked to inflammation and tissue remodeling, YKL-40 is a potential biomarker for assessing atherosclerotic disease progression. |
| FGF-2 | Fibroblast Growth Factor 2, important for angiogenesis and wound healing, implicated in vascular remodeling and atherosclerotic plaque development. |
| HSP60 | Heat Shock Protein 60, a chaperone involved in stress response, elevated levels indicate cellular stress and inflammation related to atherosclerosis. |
| IL-6 | Interleukin 6, a pro-inflammatory cytokine that plays a significant role in inflammation and is a key mediator in the atherosclerotic process. |
| IL-8 (CXCL8) | A chemokine that attracts neutrophils to sites of inflammation, its levels are associated with the inflammatory state of atherosclerotic plaques. |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1, a receptor involved in endothelial dysfunction and inflammation, linked to plaque formation. |
| Myeloperoxidase (MPO) | An enzyme produced by activated neutrophils, MPO is a marker of inflammation and oxidative stress in cardiovascular disease. |
| PAI-1 (Serpin) | Plasminogen Activator Inhibitor-1, plays a role in the regulation of fibrinolysis, its elevated levels are associated with atherothrombosis. |
| Tenascin-C | An extracellular matrix protein involved in tissue remodeling and inflammation, serves as a potential marker for atherosclerotic lesions. |
| TRAIL | TNF-related apoptosis-inducing ligand, involved in regulating apoptosis in vascular cells, potentially influencing plaque stability. |
How sensitive is the Luminex assay in detecting low-abundance biomarkers?
The Luminex assay employed in the Human Atherosclerosis Panel is highly sensitive, capable of detecting low-abundance proteins and cytokines, which are critical in early atherosclerotic processes. This high sensitivity is due to the bead-based technology, which enhances signal amplification. The limit of detection varies depending on the specific biomarker, but it generally detects concentrations in the picogram/milliliter range, ensuring reliable data even from scarce or diluted samples.
What are the potential limitations of using plasma over serum for biomarker detection?
While both plasma and serum are compatible sample types, each comes with considerations. Plasma, collected with anticoagulants (such as EDTA or citrate), may retain clotting factors that can interfere with certain assays, potentially leading to variations in biomarker detection, especially for proteins involved in coagulation. Serum, which is free of clotting factors, may offer more consistent results for certain inflammatory and metabolic markers. However, plasma is preferred when anticoagulant-sensitive biomarkers need to be assessed. Researchers should select the sample type that aligns best with their study design and target analytes.
How are results from the Human Atherosclerosis Panel typically analyzed?
Data generated from the Luminex assay is analyzed using specialized software, which translates the raw fluorescence intensities into quantitative values for each biomarker. These values are then compared to a standard curve, allowing researchers to determine the concentration of each analyte in the sample. Statistical tools such as multivariate analysis, correlation studies, and pathway enrichment analysis are commonly applied to interpret the relationships between biomarkers and to gain insights into disease mechanisms or treatment effects.
What controls are recommended for ensuring the accuracy of results in Luminex assays?
For optimal accuracy, it's crucial to include appropriate controls such as positive and negative controls, as well as internal assay controls for each biomarker being analyzed. Positive controls help verify that the assay is functioning correctly and that the biomarkers are being detected as expected. Negative controls are critical for identifying potential nonspecific binding or background signal issues. Moreover, it's advisable to use duplicate or triplicate sample wells to assess reproducibility and account for any potential variability in the assay.
How does the multiplexing capability of the Luminex assay enhance research outcomes?
The multiplexing feature of the Luminex assay significantly enhances research outcomes by allowing the simultaneous detection of multiple biomarkers in a single sample. This not only conserves sample volume but also provides a holistic view of the atherosclerotic process. Researchers can analyze various interrelated pathways, such as inflammation, lipid metabolism, and endothelial dysfunction, in one experiment, generating comprehensive datasets that enable more sophisticated analysis and a deeper understanding of the disease mechanisms.
Can the panel be used for longitudinal studies to track biomarker changes over time?
Yes, the Human Atherosclerosis Panel is well-suited for longitudinal studies. Its high sensitivity and reproducibility make it ideal for tracking changes in biomarker levels over time in experimental models or human studies. By analyzing samples collected at different time points, researchers can monitor the progression of atherosclerosis, assess the impact of interventions, or observe natural variations in biomarker expression. This dynamic approach helps in understanding disease evolution and response to treatments.

