What are T Helper 17 Cells?
T helper 17 (Th17) cells are a subset of CD4+ T helper lymphocytes characterized by their production of interleukin-17 (IL-17) family cytokines. They were first identified as a distinct subset due to their unique cytokine profile, which includes IL-17A, IL-17F, IL-17AF, and IL-22. Unlike their counterparts, Th1 and Th2 cells, Th17 cells play a crucial role in defending against extracellular pathogens, particularly at mucosal surfaces, and in modulating inflammatory responses.
Select Service
Th17 Cell Activation and Differentiation
Activation of Th17 Cells
The activation of Th17 cells is a complex, multi-step process that begins with the recognition of specific antigens by naive CD4+ T cells. This process involves several key stages and signals that drive the differentiation of naive T cells into Th17 cells.
Antigen Presentation and Initial Activation
The initial step in Th17 cell activation involves the presentation of antigens by antigen-presenting cells (APCs) such as dendritic cells and macrophages. These APCs capture and process antigens, presenting them to naive CD4+ T cells through Major Histocompatibility Complex (MHC) class II molecules. The interaction between the T cell receptor (TCR) on naive CD4+ T cells and the antigen-MHC complex is essential for the initial activation signal.
Cytokine Environment and Signal Integration
Upon recognition of the antigen, the naive T cells require additional signals provided by cytokines released by APCs. For Th17 differentiation, the cytokine environment plays a crucial role. The key cytokines involved include:
- Transforming Growth Factor-beta (TGFβ): TGFβ is critical for the initial commitment of naive T cells to the Th17 lineage. It induces the expression of the transcription factor RORγt, which is essential for Th17 differentiation.
- Interleukin-6 (IL-6): IL-6 works in concert with TGFβ to drive the differentiation process. It activates the STAT3 signaling pathway, which further enhances the expression of RORγt and promotes the production of Th17 cytokines.
- Interleukin-21 (IL-21): IL-21, secreted by activated Th17 cells themselves or by other T cell subsets, acts in an autocrine manner to sustain Th17 differentiation and function. It reinforces the Th17 phenotype by upregulating RORγt expression.
Differentiation of Th17 Cells
The differentiation of naive CD4+ T cells into Th17 cells is governed by a series of molecular and transcriptional events:
Transcription Factors and Molecular Regulators
- RORγt (Retinoic Acid Receptor-Related Orphan Receptor gamma t): RORγt is the master transcription factor driving Th17 cell differentiation. It directly promotes the expression of Th17-specific cytokines such as IL-17A and IL-17F. The presence of RORγt is a hallmark of Th17 cells and is critical for their development.
- RORα (Retinoic Acid Receptor-Related Orphan Receptor alpha): RORα, another member of the nuclear receptor family, also contributes to Th17 differentiation. It works synergistically with RORγt to regulate the Th17 gene expression program. Deficiency in both RORγt and RORα results in a significant impairment of Th17 cell development.
- IRF4 (Interferon Regulatory Factor 4): IRF4 acts upstream of RORγt. It facilitates the expression of RORγt by binding to its promoter regions. The presence of IRF4 is crucial for the full development of Th17 cells.
- BATF (Basic Leucine Zipper ATF-Like Transcription Factor): BATF is essential for the generation of Th17 cells. It interacts with RORγt and IRF4 to coordinate the Th17 differentiation process. Although BATF is not exclusive to Th17 cells, its presence is vital for the expression of Th17-associated cytokines.
- AHR (Aryl Hydrocarbon Receptor): AHR is a nuclear receptor involved in various immune processes. In Th17 cells, AHR influences the production of IL-22, although it does not affect the differentiation process itself.
Cytokine Signaling and Feedback Mechanisms
The differentiation process is regulated by positive and negative feedback mechanisms. The primary cytokines involved are:
- IL-17A and IL-17F: These are the hallmark cytokines of Th17 cells. They are critical for mediating the effects of Th17 cells, such as promoting inflammation and recruiting neutrophils to sites of infection or injury.
- IL-23: IL-23, produced by APCs, is essential for the maintenance and proliferation of Th17 cells. It acts on Th17 cells to sustain their activity and cytokine production. The IL-23 receptor (IL-23R) is upregulated during Th17 cell activation and is a crucial factor for their long-term persistence and function.
Th17 Cell Plasticity and Interactions
Th17 cells exhibit a high degree of plasticity and can adapt to different environmental cues. This plasticity is evident in their ability to trans-differentiate into other T helper cell subsets under certain conditions:
- Th1/Th17 Plasticity: Th17 cells can convert to a Th1-like phenotype in the presence of certain cytokines, such as IFNγ. This plasticity reflects their capacity to adapt to different immune challenges.
- Th17/Treg Balance: Th17 cells and T regulatory cells (Tregs) share a common developmental pathway influenced by TGFβ. The balance between Th17 and Tregs is crucial for maintaining immune homeostasis and preventing autoimmunity. Tregs can inhibit Th17 cell function and vice versa, modulating the overall immune response.
What is the Role of Th17 Cells?
Host Defense Against Pathogens
Barrier Protection: Th17 cells are especially important in protecting mucosal surfaces, including those in the gastrointestinal tract, respiratory tract, and skin. They contribute to the defense against a variety of pathogens, including bacteria, fungi, and parasites. By secreting cytokines like IL-17A and IL-17F, Th17 cells enhance the local immune response, promoting the recruitment and activation of neutrophils and other immune cells to sites of infection.
Induction of Antimicrobial Peptides: IL-17 produced by Th17 cells stimulates epithelial cells to produce antimicrobial peptides, such as β-defensins and cathelicidins. These peptides act as a first line of defense by directly killing or inhibiting the growth of pathogens.
Inflammatory Response: Th17 cells promote inflammation through the secretion of pro-inflammatory cytokines, including IL-17A, IL-17F, and IL-22. This inflammatory response is essential for clearing infections but can also contribute to tissue damage if not tightly regulated.
Regulation of Immune Responses
Modulation of Inflammation: Th17 cells play a role in balancing immune responses. They interact with other immune cells, including macrophages, dendritic cells, and T regulatory cells (Tregs), to modulate the intensity and duration of the inflammatory response. By producing IL-22, Th17 cells help to maintain the integrity of epithelial barriers and regulate inflammation, thereby preventing excessive tissue damage.
Crosstalk with Other T Helper Subsets: Th17 cells can influence the differentiation and function of other T helper cell subsets, such as Th1 and Th2 cells. This interaction is critical in shaping the overall immune response and determining the outcome of various immune challenges. For example, Th17 cells can interact with Th1 cells to enhance the production of IFNγ, further amplifying the immune response against certain pathogens.
Involvement in Autoimmune Diseases
Pathogenesis of Autoimmunity: While Th17 cells are essential for protecting against infections, their dysregulation is linked to the development of autoimmune diseases. In conditions such as rheumatoid arthritis, psoriasis, and multiple sclerosis, Th17 cells contribute to disease pathology through excessive or inappropriate inflammation. The cytokines produced by Th17 cells, particularly IL-17A and IL-17F, drive the production of pro-inflammatory cytokines and recruit immune cells that perpetuate tissue damage.
Th17/Treg Balance: The balance between Th17 cells and Tregs is crucial for maintaining immune homeostasis. An imbalance, with an overabundance of Th17 cells or a deficiency in Tregs, can lead to autoimmune diseases. Tregs normally suppress excessive Th17 responses, but when Tregs are impaired or functionally compromised, Th17 cells can become hyperactive, contributing to autoimmune pathology.
Impact on Tissue Repair and Remodeling
Wound Healing: Th17 cells, particularly through IL-22, play a role in tissue repair and remodeling. IL-22 promotes the proliferation and survival of epithelial cells, aiding in the repair of damaged tissues. This function is particularly important in the context of chronic infections or injuries, where efficient tissue regeneration is necessary for maintaining barrier integrity.
Fibrosis and Chronic Inflammation: While Th17 cells contribute to tissue repair, their persistent activation can lead to fibrosis, a condition characterized by excessive connective tissue formation. Chronic Th17-driven inflammation can result in tissue scarring and organ dysfunction, illustrating the dual role of Th17 cells in both healing and disease.
What is the Activity of Th17 Cells?
Th17 cells exert their effects primarily through cytokine secretion. The key activities of Th17 cells include:
Induction of Inflammation: IL-17A and IL-17F promote inflammation by inducing the production of pro-inflammatory cytokines (e.g., TNFα, IL-1β, IL-6) and chemokines (e.g., IL-8) from various cell types. This leads to the recruitment of neutrophils and other immune cells to sites of infection or inflammation.
Tissue Repair and Wound Healing: IL-22, produced by Th17 cells, plays a role in mucosal protection and repair. It stimulates the production of antimicrobial peptides and supports epithelial cell regeneration.
Interaction with Other Immune Cells: Th17 cells interact with other immune cell types, including T regulatory cells (Tregs). The balance between Th17 and Treg cells
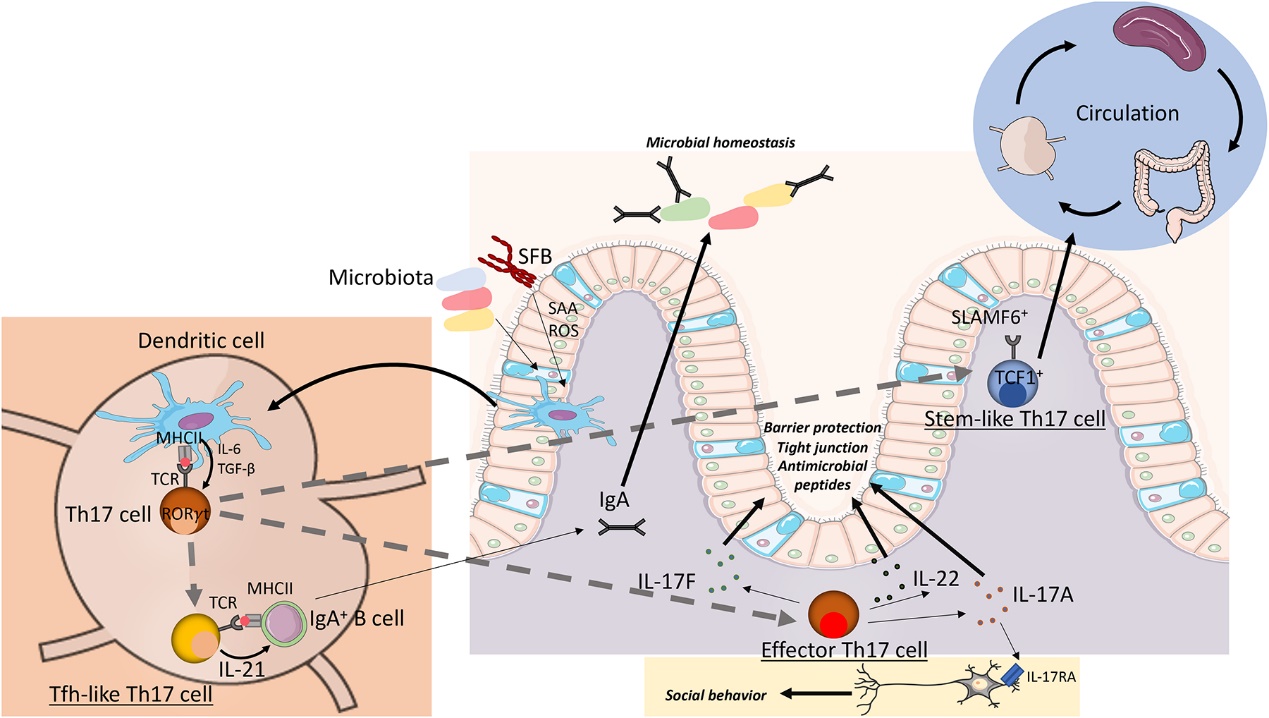 Th17 cell heterogeneity in intestinal homeostasis (Schnell et dl., 2023)
Th17 cell heterogeneity in intestinal homeostasis (Schnell et dl., 2023)
Th1 vs. Th2 vs. Th17
| Feature | Th1 Cells | Th2 Cells | Th17 Cells |
|---|---|---|---|
| Key Cytokines | IFN-γ, TNF-α, IL-2 | IL-4, IL-5, IL-13 | IL-17A, IL-17F, IL-22 |
| Primary Functions | - Mediates cellular immunity | - Mediates humoral immunity | - Protects mucosal surfaces and promotes inflammation |
| Target Pathogens | - Intracellular pathogens (e.g., viruses, some bacteria) | - Extracellular pathogens (e.g., parasites, allergens) | - Extracellular pathogens (e.g., bacteria, fungi) |
| Major Transcription Factors | T-bet | GATA-3 | RORγt |
| Key Cell Interactions | - Activates macrophages | - Stimulates B cells to produce antibodies | - Enhances neutrophil recruitment and activation |
| Inflammatory Role | - Promotes chronic inflammation and tissue damage | - Associated with allergic reactions and chronic inflammation | - Drives acute and chronic inflammation |
| Role in Autoimmunity | - Plays a role in autoimmune diseases like Type 1 Diabetes and Multiple Sclerosis | - Involved in autoimmune diseases like Asthma and Eosinophilic Esophagitis | - Implicated in autoimmune diseases like Rheumatoid Arthritis and Psoriasis |
| Response to IL-23 | - Not significantly influenced | - Not significantly influenced | - Critical for maintenance and expansion |
| Differentiation Cytokines | - IL-12, IFN-γ | - IL-4, IL-13 | - TGF-β, IL-6 |
| Function in Tissue Repair | - Less prominent | - Less prominent | - Contributes to tissue repair and remodeling |
| Associated Diseases | - Chronic inflammatory conditions (e.g., Crohn’s disease) | - Allergic diseases (e.g., asthma, eczema) | - Autoimmune diseases (e.g., rheumatoid arthritis, psoriasis) |
| Interaction with Tregs | - Often antagonistic | - Can support Tregs but can also be antagonistic | - Interaction with Tregs is complex, sometimes antagonistic |
Major Th17 Cytokines
The primary cytokines secreted by Th17 cells include IL-17A, IL-17F, IL-17AF, and IL-22. Each of these cytokines has distinct functions and mechanisms of action, contributing to both protective and pathological immune responses.
IL-17A
IL-17A is one of the hallmark cytokines produced by Th17 cells. It is a pro-inflammatory cytokine that plays a significant role in the immune system. IL-17A is involved in various biological processes, including inflammation and host defense. It promotes the production of other pro-inflammatory cytokines and chemokines that recruit and activate neutrophils and macrophages to sites of infection or injury. By promoting the production of antimicrobial peptides, IL-17A helps control infections at mucosal surfaces and other barrier tissues. IL-17A acts on epithelial cells to induce the expression of antimicrobial peptides, such as β-defensins and cathelicidins, enhancing the epithelial barrier function and host defense. In fibroblasts, IL-17A stimulates the production of extracellular matrix components and cytokines that support tissue remodeling and repair, but excessive activation can lead to fibrosis and tissue damage.
IL-17F
IL-17F shares many similarities with IL-17A, including its role in inflammation and immune defense. Both IL-17A and IL-17F are produced by Th17 cells and have similar receptor usage and signaling pathways. They both activate the same downstream signaling pathways, including NF-κB and MAPK. While IL-17A is typically more potent in its pro-inflammatory effects, IL-17F tends to have a more localized effect and is less effective in inducing inflammation compared to IL-17A. IL-17F contributes to inflammatory responses, albeit to a lesser extent than IL-17A. It plays a role in recruiting and activating immune cells to sites of inflammation and helps maintain mucosal immunity. IL-17A and IL-17F often act synergistically, leading to a stronger inflammatory response and more robust activation of target cells, enhancing the overall immune response.
IL-17AF
IL-17AF is a heterodimeric cytokine composed of one subunit each of IL-17A and IL-17F. This combination results in unique biological properties. IL-17AF is formed by the association of IL-17A and IL-17F, resulting in a cytokine with distinct receptor-binding and signaling characteristics compared to its individual components. IL-17AF has been shown to have a broad range of activities, including promoting inflammation and immune responses. It is capable of inducing the expression of a wide array of inflammatory mediators and antimicrobial peptides. IL-17AF contributes to the recruitment of immune cells and the production of pro-inflammatory cytokines, similar to IL-17A and IL-17F. This cytokine can exacerbate inflammatory diseases and contribute to the pathology of autoimmune conditions by enhancing the inflammatory response and tissue damage. IL-17AF often exhibits enhanced inflammatory effects compared to IL-17A or IL-17F alone, inducing more robust expression of inflammatory mediators and has been linked to more severe inflammatory responses in certain disease contexts.
IL-22
IL-22 is a cytokine that, while produced by Th17 cells, has distinct roles from other Th17 cytokines. IL-22 plays a critical role in maintaining mucosal barrier integrity and enhancing the epithelial response to infection. It helps to protect epithelial cells from damage and promotes wound healing. By stimulating epithelial cells to proliferate and produce antimicrobial peptides, IL-22 supports tissue repair and recovery following injury or infection. IL-22 production is closely linked to IL-23 and IL-6, which are crucial for the differentiation and maintenance of Th17 cells. IL-23 enhances the stability and expansion of Th17 cells, while IL-6 contributes to their differentiation and function. The presence of IL-23 and IL-6 in the microenvironment can influence the production of IL-22, further affecting mucosal immunity and tissue repair processes. IL-22 primarily acts on non-hematopoietic cells, including epithelial cells and fibroblasts, inducing the production of antimicrobial peptides and supporting barrier function in epithelial tissues. IL-22 enhances the antimicrobial defense mechanisms at mucosal surfaces, playing a role in protecting against various pathogens and maintaining tissue homeostasis.
IL-21
IL-21 is a pleiotropic cytokine produced by various T cells, including Th17 cells. It plays a critical role in the autocrine and paracrine regulation of Th17 cells by promoting their differentiation, survival, and function. IL-21 enhances the expression of Th17-related transcription factors and cytokines, contributing to the stability and expansion of the Th17 cell population. Additionally, IL-21 supports the function of other immune cells, including B cells, CD8+ T cells, and NK cells, thereby enhancing overall immune responses.
GM-CSF
GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) is another important cytokine produced by Th17 cells. GM-CSF plays a vital role in the activation and function of various immune cells, including macrophages and dendritic cells. It promotes the survival, proliferation, and differentiation of these cells, enhancing their ability to present antigens and produce inflammatory cytokines. GM-CSF also contributes to the recruitment and activation of neutrophils, further amplifying the inflammatory response.
IL-26
IL-26 is a member of the IL-10 cytokine family and is produced by Th17 cells. It has been shown to have antimicrobial properties and can enhance the production of pro-inflammatory cytokines by other immune cells. IL-26 contributes to mucosal immunity by directly acting on epithelial cells to promote the secretion of antimicrobial peptides and enhance barrier function.
| Species | Description | Analytes | |
|---|---|---|---|
| Human | Human Th17 15-plex Panel | IL-1β, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, IL-31, IL-33, IFN-γ, sCD40L, TNF-α. | +Inquiry |
| Human Th17 25-plex Panel | IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17A/CTLA8, IL-17E/IL-25, IL-17F, IL-1β, IL-2, IL-21, IL-22, IL-23, IL-27, IL-28A/IFNλ2, IL-31, IL-33, IFN-, GM-CSF, MIP-3α/CCL20, TNFα, TNFβ | +Inquiry | |
| Mouse | Mouse Th17 6-Plex Panel | IL-1β, IL-4, IL-6, IL-10, IL-17A, IFN-γ, TNF-α. | +Inquiry |
| Mouse Th17 8-Plex Panel | CD40L, IL-17F, IL-21, IL-22, IL-23p19, IL-31, IL-33, MIP-3α | +Inquiry | |
| Mouse Th17 25-Plex Panel | L-4, IL-5, IL-6, IL-10, IL-12 (p70), L-13, L-15, IL-17A/CTLA8, L-17E/IL-25, IL-17F, IL-1β, IL-2, IL-21, IL-22, IL-23, IL-27, IL-28B/IFNλ3, IL-31, IL-33, IFNγ, GM-CSF, MIP-3α/CCL20, sCD40L, TNFα, TNFβ | +Inquiry |
Luminex Multiplex Assays for Studying Th17
Luminex multiplex assays represent a powerful tool for the simultaneous quantification of multiple cytokines and proteins, offering significant advantages in the study of Th17 cell biology. These assays utilize bead-based technology combined with flow cytometry, enabling the detection and analysis of various soluble factors in a single sample. This high-throughput capability is particularly valuable in elucidating the complex cytokine milieu associated with Th17 cells.
Luminex multiplex assays are extensively used in both basic and clinical research to explore the role of Th17 cells in health and disease. In autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, these assays help identify cytokine profiles associated with disease severity and progression. Similarly, in infectious diseases, Luminex assays can reveal how Th17 cells contribute to pathogen clearance and immune regulation.
In cancer research, Luminex assays facilitate the study of the tumor microenvironment, where Th17 cells can have either pro- or anti-tumor effects. By analyzing the cytokine milieu within tumors, we can gain insights into how Th17 cells influence cancer progression and response to therapy.
Reference:
- Schnell, Alexandra, Dan R. Littman, and Vijay K. Kuchroo. "TH17 cell heterogeneity and its role in tissue inflammation." Nature immunology 24.1 (2023): 19-29.