The immune system is a sophisticated network of cells, tissues, and organs dedicated to safeguarding the body from pathogens, toxins, and malignancies. It operates through a complex interplay of innate and adaptive responses to detect and neutralize threats. Central to this defense mechanism are T cells, a pivotal component of the adaptive immune system. These cells are not only responsible for recognizing specific antigens but also for orchestrating a targeted response to ensure effective immune protection.
T Helper Cells
T helper (Th) cells are a specialized subset of CD4+ T lymphocytes crucial for orchestrating the adaptive immune response. They originate from the thymus and, upon activation, differentiate into various subsets that tailor the immune response to different types of pathogens. Two of the most well-studied subsets are Th1 and Th2 cells, each playing a distinct role in immune regulation and defense.
Th1 Cells
Definition and Characteristics of Th1 Cells
Th1 cells are integral to the cellular immune response, primarily defending against intracellular pathogens such as viruses and certain bacteria. These cells are characterized by their production of key cytokines, including interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). Th1 differentiation is driven by the transcription factor T-bet, which promotes the development of Th1 cells from naive CD4+ T cells.
The primary function of Th1 cells is to enhance the ability of macrophages to phagocytize and kill intracellular pathogens. This is achieved through the release of IFN-γ, which activates macrophages and increases the expression of major histocompatibility complex (MHC) molecules, enhancing antigen presentation. Th1 cells also contribute to the activation of cytotoxic T lymphocytes (CTLs), which are critical for eliminating infected cells.
Cytokines and Signaling Molecules
Th1 cells are distinguished by their production of specific cytokines that shape their function and influence other immune cells. Key cytokines include:
- Interferon-Gamma (IFN-γ): A potent cytokine that activates macrophages, enhances antigen presentation, and induces the production of antimicrobial peptides.
- Tumor Necrosis Factor-Alpha (TNF-α): Involved in inflammation and the regulation of immune responses, TNF-α also plays a role in the apoptosis of infected cells.
- Interleukin-2 (IL-2): Promotes the proliferation of T cells and enhances the cytotoxic activity of CTLs.
The differentiation of Th1 cells is regulated by the JAK-STAT signaling pathway, particularly through STAT1 and STAT4, which are activated in response to IFN-γ and other cytokines.
Th1 in Disease
Autoimmune Diseases: An overactive Th1 response can lead to autoimmune diseases, where the immune system erroneously targets the body's own tissues. Conditions such as Type 1 Diabetes Mellitus and Multiple Sclerosis are associated with excessive Th1 activity, leading to chronic inflammation and tissue damage.
Chronic Infections: Th1 cells are essential in managing chronic infections like tuberculosis (TB). While a robust Th1 response helps control TB, excessive or prolonged activation can result in granuloma formation and persistent inflammation, complicating disease management.
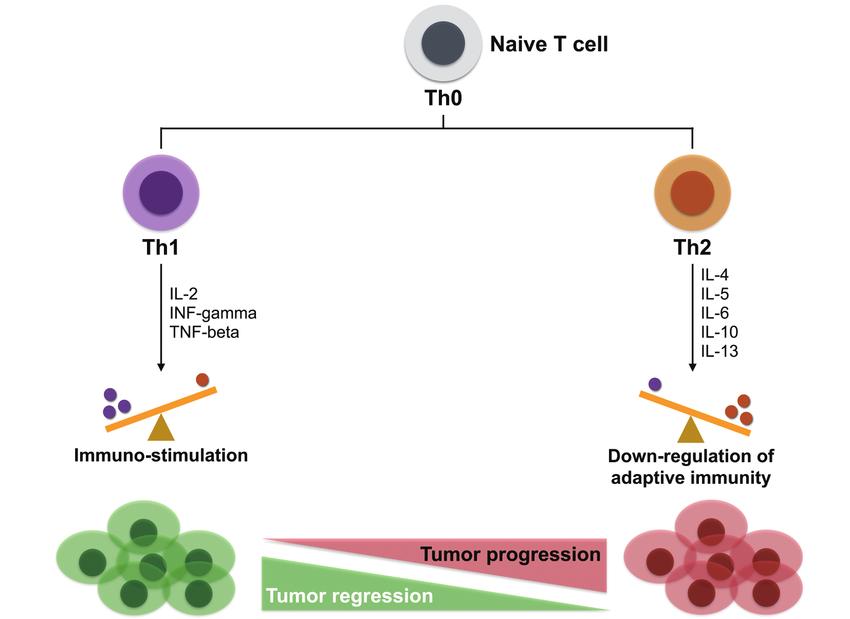 Effects of Th1/Th2 on tumor progression (Lin et al., 2017).
Effects of Th1/Th2 on tumor progression (Lin et al., 2017).
Th2 Cells
Definition and Characteristics of Th2 Cells
Th2 cells are pivotal in the humoral immune response, particularly against extracellular pathogens like parasites and allergens. They are defined by their production of cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13). The differentiation of Th2 cells is driven by the transcription factor GATA3, which directs naive CD4+ T cells towards the Th2 lineage.
Th2 cells facilitate the activation of B cells, promoting antibody production and class switching to immunoglobulin E (IgE). This is crucial for the immune response against parasitic infections and for mediating allergic reactions. Th2 cells also recruit eosinophils and other immune cells to the site of infection or inflammation, contributing to the overall immune response.
Cytokines and Signaling Molecules
Th2 cells secrete a distinct set of cytokines that orchestrate their role in immunity:
- Interleukin-4 (IL-4): Stimulates B cell proliferation, differentiation, and class switching to IgE, which is critical in allergic responses.
- Interleukin-5 (IL-5): Promotes the growth and activation of eosinophils, which are involved in combating parasitic infections and in allergic inflammation.
- Interleukin-13 (IL-13): Enhances mucus production and contributes to tissue remodeling, playing a significant role in allergic inflammation and asthma.
The Th2 response is mediated through the IL-4R and IL-13R signaling pathways, with STAT6 being a key transcription factor involved in Th2 differentiation and function.
Th2 in Disease
Allergic Diseases: Th2 cells are central to the development of allergic conditions such as asthma and allergic rhinitis. The cytokines produced by Th2 cells drive IgE production, leading to hypersensitivity reactions and chronic inflammation in target tissues.
Parasitic Infections: Th2 responses are effective in managing helminth infections. The Th2 cytokines help orchestrate a response that includes the expulsion of parasites and modulation of the immune response to prevent excessive tissue damage.
Balance of Th1/Th2
The balance between Th1 and Th2 responses is crucial for maintaining immune homeostasis and ensuring an effective defense against various pathogens while preventing excessive inflammation or autoimmunity. This balance is achieved through a delicate interplay of cellular signaling, cytokine production, and regulatory mechanisms. An imbalance in this equilibrium can lead to pathological conditions, manifesting either as an overactive Th1 response, which may result in autoimmune diseases, or an overactive Th2 response, which can lead to allergic disorders.
The Th1/Th2 balance is regulated by the differential expression of transcription factors and cytokines. Th1 cells primarily produce cytokines that promote cellular immunity and inflammation, while Th2 cells produce cytokines that facilitate humoral immunity and suppress Th1 responses. The dynamic equilibrium between these subsets is vital for adapting the immune response to different types of pathogens and maintaining tolerance to self-antigens.
Mechanisms of Balancing
Several mechanisms are involved in balancing Th1 and Th2 responses. These include:
- Cytokine Feedback Loops: Cytokines produced by Th1 and Th2 cells can influence the differentiation and function of each other. For example, IFN-γ produced by Th1 cells can inhibit Th2 differentiation, while IL-4 from Th2 cells can suppress Th1 responses.
- Transcription Factors: Transcription factors such as T-bet and GATA3 drive the differentiation of Th1 and Th2 cells, respectively. The expression of these factors is regulated by various signaling pathways and cytokine interactions, ensuring a balanced Th1/Th2 response.
- Cross-Regulation: The interaction between Th1 and Th2 cells involves cross-regulation, where the cytokines produced by one subset can inhibit the activity of the other. This cross-talk is crucial for preventing an overactive immune response and maintaining homeostasis.
Regulatory Mechanisms of Th1 and Th2
Genetic Factors
Genetic predisposition plays a significant role in shaping the Th1/Th2 balance. Specific genetic polymorphisms can influence the development and function of Th1 and Th2 cells. For instance, variations in genes encoding cytokines and their receptors, as well as transcription factors like T-bet and GATA3, can impact the susceptibility to Th1- or Th2-mediated diseases. Understanding these genetic influences can provide insights into individual variations in immune responses and disease susceptibility.
Environmental Influences
Environmental factors such as infections, diet, and exposure to allergens can significantly affect the Th1/Th2 balance.
- Infections: Early-life infections can skew the immune response towards a Th1 or Th2 predominance. For example, exposure to certain bacterial infections may promote a Th1-biased response, while viral infections can influence Th2 responses.
- Diet: Nutritional factors can modulate the Th1/Th2 balance. For instance, dietary components such as fatty acids and vitamins can influence cytokine production and immune cell differentiation, thereby affecting the Th1/Th2 equilibrium.
- Allergen Exposure: Exposure to allergens can drive a Th2-skewed response, leading to allergic diseases. Chronic exposure to allergens can perpetuate this imbalance, contributing to persistent allergic inflammation.
Regulatory Cells
Regulatory T cells (Tregs) and other regulatory mechanisms play a pivotal role in modulating Th1 and Th2 responses. Tregs, characterized by the expression of the FOXP3 transcription factor, help maintain immune tolerance and prevent excessive inflammatory responses. They achieve this by:
- Suppressing Effector T Cell Activity: Tregs can inhibit the activation and proliferation of Th1 and Th2 cells through direct cell contact or secretion of anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta (TGF-β).
- Modulating Cytokine Production: Tregs influence cytokine production by Th1 and Th2 cells, thus helping to maintain a balanced immune response. By secreting cytokines like IL-10, Tregs can dampen the inflammatory effects of Th1 cells, while TGF-β can inhibit Th2 responses.
- Maintaining Immune Tolerance: Tregs play a crucial role in preventing autoimmunity by ensuring that self-reactive T cells are kept in check. This function is essential for preserving the balance between Th1 and Th2 responses and preventing autoimmune disease development.
What is The Difference Between Th1 and Th2 Markers?
| Characteristic | Th1 | Th2 |
|---|---|---|
| Primary Cytokines | IFN-γ (Interferon-gamma) | IL-4 (Interleukin-4) |
| TNF-α (Tumor Necrosis Factor-alpha) | IL-5 (Interleukin-5) | |
| IL-2 (Interleukin-2) | IL-13 (Interleukin-13) | |
| Transcription Factors | T-bet (T-box expressed in T cells) | GATA3 (GATA-binding protein 3) |
| STAT4 (Signal Transducer and Activator of Transcription 4) | STAT6 (Signal Transducer and Activator of Transcription 6) | |
| Functions | Promotes cellular immunity | Promotes humoral immunity |
| Activates macrophages | Activates B cells | |
| Enhances cytotoxic T lymphocyte (CTL) activity | Promotes IgE production | |
| Defends against intracellular pathogens (viruses, bacteria) | Defends against extracellular pathogens (parasites, allergens) | |
| Involved in autoimmune diseases | Involved in allergic diseases | |
| Associated Diseases | Multiple Sclerosis, Type 1 Diabetes Mellitus, Rheumatoid Arthritis | Asthma, Allergic Rhinitis, Eczema |
| Regulatory Cytokines | IL-12 (drives differentiation) | IL-4 (drives differentiation) |
| IL-18 | IL-10 | |
| IL-27 | IL-25 | |
| Immune Response Type | Pro-inflammatory | Anti-inflammatory (in the context of inhibiting Th1 responses) |
| Primary Role | Clears infections through promoting cell-mediated immunity | Clears infections through promoting antibody-mediated immunity |
| Marker Expression | CD183 (CXCR3) | CD194 (CCR4) |
| CD119 (IFN-γ receptor) | CD294 (CRTH2) | |
| CD256 (LIGHT) | CD30 | |
| Regulatory Cells | Regulatory T cells (Tregs) | Regulatory T cells (Tregs) |
| Intercellular Interactions | Th1 cells interact with macrophages and CTLs | Th2 cells interact with B cells and eosinophils |
| Enhance phagocytosis via IFN-γ and TNF-α | Promote antibody production and mucus secretion via IL-4 and IL-13 | |
| Pathological Mechanisms | Cell-mediated cytotoxicity and inflammatory responses | Humoral hypersensitivity and inflammatory responses |
Th1/Th2 Analysis Using Luminex xMAP Technology
Understanding the balance and regulation of Th1 and Th2 immune responses is critical for investigating various immune-mediated diseases and developing targeted therapies. Luminex xMAP technology offers a powerful and versatile platform for analyzing multiple cytokines simultaneously, providing insights into the Th1/Th2 balance and associated immune responses.
Luminex xMAP (Multi-Analyte Profiling) technology is a bead-based multiplexing platform that enables the simultaneous detection and quantification of multiple analytes in a single sample. The technology combines the principles of flow cytometry with advanced bead-based immunoassays, allowing for high-throughput, sensitive, and specific measurement of cytokines and other biomarkers.
Select Service
Applications in Th1/Th2 Analysis
Luminex xMAP technology allows for comprehensive cytokine profiling, facilitating the simultaneous measurement of multiple Th1 and Th2 cytokines in a single assay. This capability is crucial for understanding the balance between Th1 and Th2 responses in various contexts, such as autoimmune diseases, infections, and allergic conditions.
Immune Response Characterization
By quantifying Th1 and Th2 cytokines, researchers can characterize the immune response in different disease states. For instance, a predominance of Th1 cytokines (e.g., IFN-γ, TNF-α) may indicate a Th1-skewed response, often associated with autoimmune diseases and chronic infections. Conversely, elevated levels of Th2 cytokines (e.g., IL-4, IL-5, IL-13) suggest a Th2-skewed response, commonly seen in allergic diseases and parasitic infections.
Biomarker Discovery
The high-throughput nature of Luminex xMAP technology enables the identification of novel biomarkers associated with Th1 and Th2 responses. This can aid in the discovery of new therapeutic targets and the development of diagnostic tools for immune-mediated diseases.
Advantages of Luminex xMAP Technology
- Multiplexing Capability: Simultaneous analysis of multiple cytokines in a single sample reduces sample volume requirements and increases throughput.
- High Sensitivity and Specificity: The use of specific capture and detection antibodies ensures accurate and reliable quantification of cytokines.
- Flexibility: Luminex xMAP technology can be adapted to analyze a wide range of biomarkers, making it suitable for various research applications.
- Efficiency: The platform allows for rapid data acquisition and analysis, facilitating large-scale studies and longitudinal monitoring of immune responses.
Reference:
- Lin, Chang-Ni, Chih-Yen Chien, and Hui-Ching Chung. "Are friends or foes? New strategy for head and neck squamous cell carcinoma treatment via immune regulation." International Journal of Head and Neck Science 1.2 (2017): 105-113.

