What Are Matrix Metalloproteinases?
Matrix metalloproteinases (MMPs) are a structurally related but functionally diverse group of zinc-dependent endopeptidases. As members of the metzincin superfamily, they share a conserved catalytic domain that binds a single Zn²⁺ ion essential for proteolytic activity, along with a structural motif that maintains enzyme stability and substrate coordination. However, their domain architecture and substrate specificities vary significantly, enabling precise control over ECM degradation and remodeling in a wide range of tissue environments.
The human genome encodes 23 MMPs, traditionally categorized into subfamilies based on their preferred substrates and domain structures:
- Collagenases (e.g., MMP-1, -8, -13): Specialize in cleaving native fibrillar collagens (type I, II, III), a unique biochemical capability critical to connective tissue turnover.
- Gelatinases (MMP-2 and MMP-9): Degrade denatured collagens and gelatin, but also target basement membrane components such as laminin and entactin. These enzymes contain fibronectin type II-like inserts that enhance substrate binding.
- Stromelysins (MMP-3, -10, -11): Display broad substrate specificity but do not cleave interstitial collagens; instead, they act on proteoglycans, fibronectin, and laminin.
- Membrane-type MMPs (MT-MMPs) (e.g., MMP-14, -15): These are either anchored to the cell membrane via transmembrane domains or glycosylphosphatidylinositol (GPI) anchors, enabling pericellular matrix remodeling and proteolytic activation of other MMPs.
- Others (e.g., MMP-12/macrophage elastase, MMP-20/enamelysin): Display specialized activity in tissue-specific contexts, including elastin degradation in lung tissue or enamel matrix processing during tooth development.
Each MMP is synthesized as an inactive zymogen (proMMP), with a pro-domain masking the active site. The signature "cysteine switch" mechanism prevents premature activation: a conserved cysteine residue coordinates the catalytic zinc ion, maintaining latency until the inhibitory bond is disrupted.
Phylogenetic analysis suggests that MMP diversification has co-evolved with the complexity of vertebrate ECM architecture. This specialization allows tissues to modulate mechanical properties and biochemical cues with high spatial and temporal resolution — a feature increasingly leveraged in organoid systems, microfluidic models, and matrix engineering studies.
Matrix Metalloproteinase Structure
Matrix metalloproteinases share a modular structure that governs their enzymatic function, substrate specificity, and regulation. The canonical MMP architecture comprises three principal domains:
- Pro-domain: This N-terminal region maintains the enzyme in an inactive zymogen (proMMP) state. It contains a conserved cysteine residue that coordinates with the catalytic zinc ion in the active site, a mechanism known as the "cysteine switch," which physically blocks substrate access and prevents premature proteolysis.
- Catalytic domain: Central to MMP activity, this domain houses the Zn²⁺ ion essential for peptide bond hydrolysis, coordinated by three histidine residues within the highly conserved HEXXHXXGXXH motif. The catalytic domain's topology includes a substrate-binding cleft, the structural features of which dictate substrate specificity and enzyme kinetics.
- Hemopexin-like C-terminal domain: Present in most MMPs except minimal-domain enzymes like MMP-7, this domain facilitates substrate recognition and interaction with tissue inhibitors of metalloproteinases (TIMPs). It also participates in protein-protein interactions essential for localization and activation.
Some MMPs include additional domains, such as fibronectin type II inserts in gelatinases (MMP-2, MMP-9), which enhance binding affinity for denatured collagens and gelatin substrates. Membrane-type MMPs (MT-MMPs) possess transmembrane or glycosylphosphatidylinositol (GPI) anchors that tether them to the cell surface, enabling localized pericellular proteolysis and activation of other proMMPs.
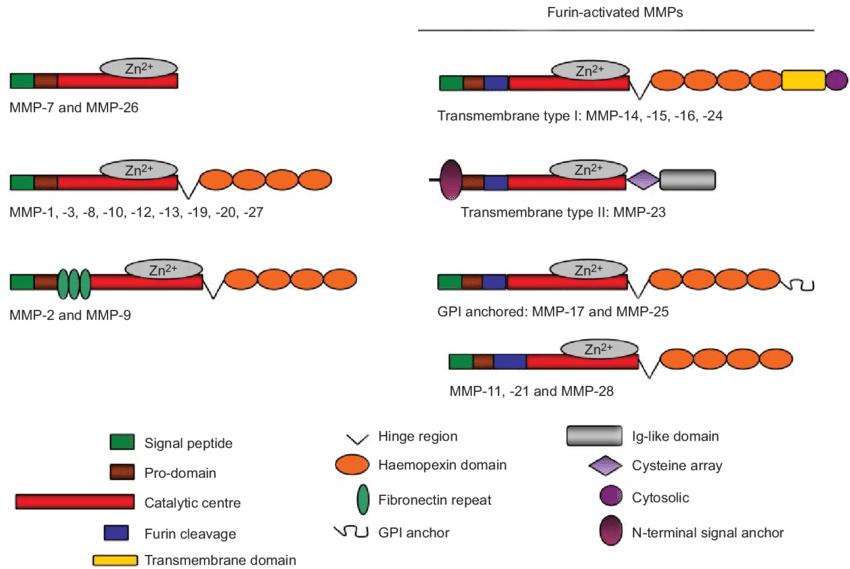 Domain structure of matrix metalloproteinase (MMP) groups (Löffek et al., 2011).
Domain structure of matrix metalloproteinase (MMP) groups (Löffek et al., 2011).
Cellular Sources of Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are expressed and secreted by a wide range of cell types under both physiological and pathological conditions. Their cellular origins are context-dependent and tightly regulated by the microenvironment, cytokine milieu, and cellular differentiation state. Understanding the cellular sources of MMPs is crucial for elucidating their biological functions and for interpreting multiplex assay data derived from complex biological samples such as serum, BALF, or tumor lysates.
1. Fibroblasts and Cancer-Associated Fibroblasts (CAFs):
Fibroblasts are major producers of interstitial collagenases (e.g., MMP-1, MMP-13) and gelatinases (e.g., MMP-2) in connective tissues. In the tumor microenvironment, fibroblasts often transition into activated CAFs, which exhibit elevated secretion of MMP-2, MMP-3, and MMP-9, facilitating extracellular matrix remodeling, angiogenesis, and metastatic niche formation.
2. Epithelial and Endothelial Cells:
Epithelial cells can upregulate MMP expression (e.g., MMP-7, MMP-9) in response to injury, infection, or oncogenic transformation. Endothelial cells produce MMP-2 and MMP-14 (MT1-MMP), which are essential for basement membrane degradation and neovascular sprouting during angiogenesis.
3. Immune Cells:
Activated macrophages, neutrophils, and T cells are prominent sources of inducible MMPs in inflammatory settings. Neutrophils constitutively store MMP-8 and MMP-9 in granules, releasing them rapidly upon degranulation. Macrophages can express a broad MMP repertoire (including MMP-1, -9, -12), particularly in chronic inflammation, tissue injury, and atherosclerosis. Certain MMPs, such as MMP-12, are specifically enriched in M2-polarized macrophages and play roles in tissue remodeling and resolution of inflammation.
4. Smooth Muscle and Mesenchymal Cells:
Vascular smooth muscle cells express MMPs in response to mechanical stress and pro-inflammatory cytokines, contributing to vascular remodeling and plaque instability in cardiovascular disease. Mesenchymal stem cells (MSCs) also express MMPs during differentiation and tissue regeneration processes.
5. Tumor Cells:
Many cancer cells aberrantly express MMPs, particularly MMP-1, MMP-7, and MMP-9, driven by oncogenic signaling pathways such as Ras/MAPK, Wnt/β-catenin, or NF-κB. These MMPs contribute to cell invasion, epithelial-to-mesenchymal transition (EMT), and immune evasion.
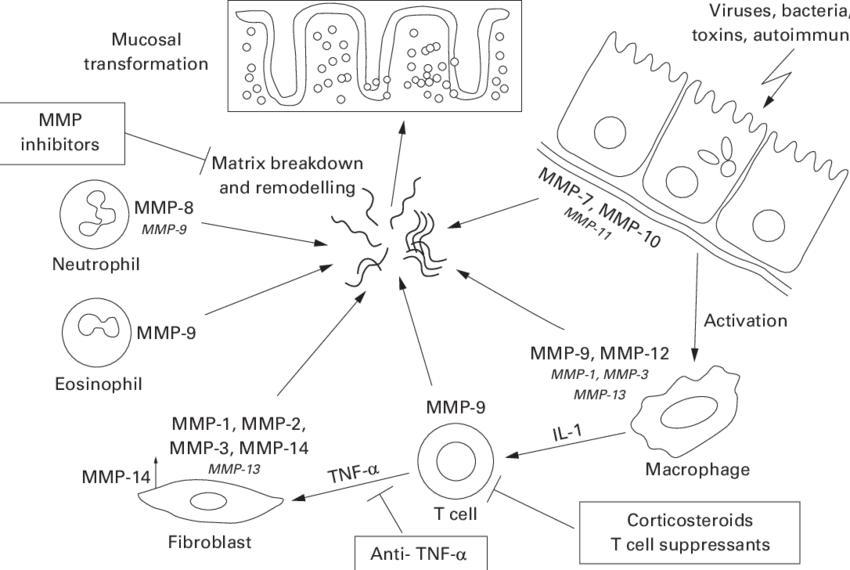 Metalloproteinases (MMPs) and their cellular sources in the intestine (Schuppan et al., 2000).
Metalloproteinases (MMPs) and their cellular sources in the intestine (Schuppan et al., 2000).
Biological Functions of MMPs
Matrix metalloproteinases serve as pivotal regulators of the extracellular matrix (ECM), orchestrating a delicate balance between matrix degradation and synthesis that is essential for tissue homeostasis. Their enzymatic activity enables the remodeling of structural proteins such as collagen, elastin, fibronectin, and laminin, which collectively form the scaffolding that defines tissue architecture and mechanical integrity. This remodeling is fundamental to numerous physiological processes, including embryonic development, where MMPs facilitate morphogenetic movements and organ formation by modulating ECM barriers.
In adult tissues, MMPs contribute to dynamic processes such as wound healing and angiogenesis. For example, during tissue repair, MMP-mediated ECM degradation clears damaged matrix components and liberates bioactive fragments—matrikines—that act as signaling molecules to recruit immune cells and stimulate new tissue growth. In angiogenesis, MMPs degrade basement membranes and perivascular ECM, allowing endothelial cells to migrate and form new capillaries, a process critical not only for normal physiology but also for tissue regeneration and tumor progression.
Beyond structural remodeling, MMPs exert significant influence on cellular signaling. By cleaving cytokines, chemokines, growth factors, and their receptors, MMPs modulate the bioavailability and activity of these molecules, thus affecting inflammation, cell proliferation, differentiation, and apoptosis. For instance, MMP-7 is known to activate pro-TNF-α, while MMP-9 can release sequestered vascular endothelial growth factor (VEGF) from the ECM, promoting angiogenic signaling cascades.
The interplay between MMPs and cellular receptors such as integrins and cadherins also modulates cell adhesion and migration. MMP-mediated cleavage of E-cadherin disrupts cell-cell junctions, facilitating epithelial-mesenchymal transition (EMT), a process essential in development and implicated in cancer metastasis. Furthermore, MMPs regulate immune cell infiltration by remodeling pericellular matrices, thereby influencing both innate and adaptive immune responses.
Importantly, the precise control of MMP activity is critical: excessive or uncontrolled MMP expression and activity contribute to pathological conditions including fibrosis, chronic inflammation, and tumor invasion. Thus, understanding the physiological roles of MMPs provides foundational insight into both tissue dynamics and disease mechanisms, underpinning their significance in biomedical research.
Activation, and Regulation of Matrix Metalloproteinase
Activation Mechanisms
MMPs are synthesized as latent zymogens (proMMPs) and require proteolytic removal of their N-terminal pro-domain to become catalytically active. This activation is tightly regulated and context-dependent, ensuring spatial and temporal control of ECM degradation. Activation may occur via:
- Other MMPs: e.g., MMP-3 can activate proMMP-9 and proMMP-1 in proteolytic cascades.
- Serine proteases: such as plasmin and trypsin.
- Non-proteolytic mechanisms: including oxidative stress (ROS), acidic pH, or S-nitrosylation that destabilize the cysteine switch, rendering the active site accessible.
For membrane-type MMPs (e.g., MMP-14), activation can occur intracellularly in the Golgi and be trafficked to the membrane already active, further enhancing their pericellular proteolytic capacity.
Regulation by TIMPs
Tissue inhibitors of metalloproteinases (TIMP-1 – TIMP-4) bind MMPs in a 1:1 ratio, but the interactions are neither random nor purely inhibitory:
| TIMP | Preferred/critical MMP partners | Functional nuance | In-vivo phenotype* |
|---|---|---|---|
| TIMP-1 | High-affinity for pro/active MMP-9; also binds MMP-1, -3 | Classic competitive inhibition; limits neutrophil-derived gelatinase bursts | Timp1-/- mice show exaggerated neuro-inflammation and faster tumor growth; TIMP-1 over-expression reduces metastasis but predisposes to liver fibrosis |
| TIMP-2 | Binds MMP-2 and MT1-MMP (MMP-14) | Dual role: (i) inhibitor of soluble MMP-2; (ii) at the cell surface acts as a bridging adaptor—TIMP-2–MT1-MMP complex recruits proMMP-2, which MT1-MMP then activates | Timp2-/- mice display enhanced angiogenic sprouting and elevated blood pressure; cardiac-specific over-expression suppresses post-MI remodeling |
| TIMP-3 | Broad ECM-bound inhibitor of MMP-1, -2, -9, ADAM17 | ECM tethering localises inhibition; also blocks TNF-α shedding | Timp3-/- mice develop emphysema-like lung defects and dilated cardiomyopathy due to unchecked ECM turnover |
| TIMP-4 | Enriched in heart, inhibits MMP-2, -9 | Modulates pericardial matrix dynamics | Timp4-/- hearts show impaired pressure-overload adaptation; over-expression reduces ventricular dilation |
*Representative phenotypes drawn from knockout or transgenic mouse studies.
Transcriptional and Post-transcriptional Regulation
MMP gene expression is tightly controlled by extracellular signals, including cytokines (e.g., TNF-α, IL-1β), growth factors, and mechanical stress. Promoter regions often contain AP-1, NF-κB, and STAT binding elements, allowing cells to integrate inflammatory and mechanical cues. Post-transcriptionally, MMP mRNA is further regulated by RNA-binding proteins, mRNA decay elements, and microRNAs (e.g., miR-29, miR-21), influencing transcript stability and translation efficiency.
Post-Translational Modifications (PTMs)
Beyond gene expression and zymogen activation, MMPs undergo several PTMs that modulate their activity, localization, and interaction profiles. Glycosylation—especially N-linked modifications—is critical for proper folding, secretion, and stability. For example, MMP-9 glycosylation affects its resistance to autolysis and interaction with extracellular components.
Phosphorylation events, while less commonly characterized, have been observed to regulate subcellular trafficking and interactions with adaptor proteins, particularly in membrane-tethered MMPs. Oxidative modifications, including S-nitrosylation or carbonylation, can either promote or impair catalytic function by altering the integrity of the Zn²⁺ coordination environment. Furthermore, additional proteolytic trimming beyond the pro-domain can either enhance or disable catalytic efficiency, depending on cleavage context.
Together, these PTMs form an essential regulatory tier that controls not just whether an MMP is active, but also how, where, and for how long it acts—thereby ensuring precise coordination with cellular and microenvironmental cues.
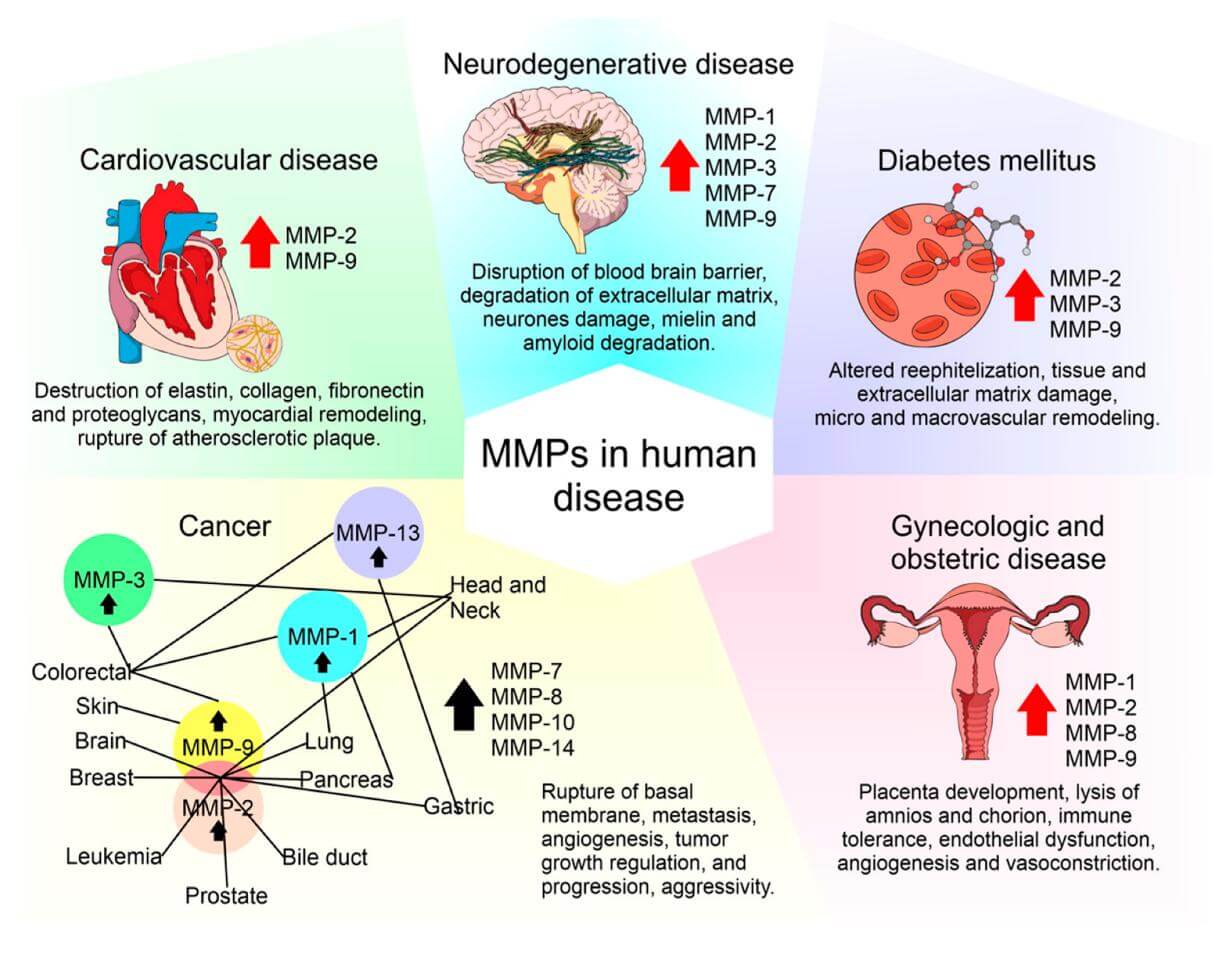 MMPs play distinguishing roles in the pathogenesis of multiple common human diseases (Cabral et al., 2020).
MMPs play distinguishing roles in the pathogenesis of multiple common human diseases (Cabral et al., 2020).
MMPs in Basic and Translational Research
Basic Research Applications
In vitro and in vivo models have been instrumental in dissecting MMP functions. Gene knockout and transgenic mouse models reveal non-redundant and isoform-specific roles. For example, MMP-14 (MT1-MMP) knockout mice display severe skeletal abnormalities and impaired angiogenesis, highlighting the enzyme’s indispensable role in developmental morphogenesis and vascular remodeling. Similarly, MMP-9 knockout models elucidate roles in immune cell migration and neuroinflammation, advancing understanding of MMPs beyond ECM degradation.
In cell culture systems, MMPs are used to study cell migration, invasion, and matrix interactions. These models enable detailed analysis of signaling pathways influenced by MMP-mediated cleavage of substrates, such as growth factors and adhesion molecules. Furthermore, engineered 3D matrices embedded with MMP-sensitive motifs allow for controlled investigations of matrix stiffness, degradation, and mechanotransduction, enriching insights into cellular responses to dynamic microenvironments.
Translational Research Implications
MMPs are extensively studied in pathological contexts like cancer, fibrosis, and chronic inflammation, where aberrant ECM remodeling contributes to disease progression. In oncology, MMP activity is associated with tumor invasion, metastasis, and angiogenesis. Multiplex MMP profiling aids in stratifying tumor aggressiveness and identifying therapeutic vulnerabilities. Additionally, MMP expression signatures inform biomarker panels for disease staging and prognosis in preclinical models.
In fibrosis research, elevated MMP-2 and MMP-9 levels correlate with excessive matrix turnover and tissue stiffening. MMPs also modulate immune cell infiltration and cytokine activation, implicating them in chronic inflammatory diseases such as rheumatoid arthritis and pulmonary fibrosis. Understanding MMP regulation in these settings is crucial for developing targeted interventions.
From a drug discovery perspective, MMP assays facilitate high-throughput screening of inhibitors and modulators, although clinical translation has been challenging due to specificity and toxicity issues. Nonetheless, advances in isoform-selective inhibition and allosteric modulation are expanding therapeutic possibilities.
Experimental Tools and Applications in Research
A comprehensive understanding of matrix metalloproteinases (MMPs) depends on sophisticated experimental tools that allow precise quantification, activity measurement, localization, and functional interrogation. Technological advances have significantly enhanced the resolution and throughput of MMP analysis, facilitating translational research across a wide range of biological systems.
Enzymatic Activity and Substrate Profiling
Quantifying MMP catalytic activity is critical for evaluating functional dynamics in both physiological and pathological settings. Gelatin zymography remains a widely used semi-quantitative method for analyzing gelatinase activity, particularly for MMP-2 and MMP-9, in tissue and cell lysates. For high-sensitivity and kinetic studies, fluorogenic substrates that mimic native cleavage motifs allow real-time detection of MMP activity in live-cell assays and tissue explants.
Activity-based probes (ABPs) have emerged as powerful tools to label active MMPs covalently, enabling in situ visualization and enrichment for downstream mass spectrometry analysis. These technologies have contributed to the emergence of degradomics—an approach focused on mapping substrate cleavage events and proteolytic networks at systems scale.
Expression Analysis and Functional Genomics
MMP expression can be quantified at both transcript and protein levels using qPCR, ELISA, Western blotting, and immunohistochemistry. For spatial resolution, in situ hybridization and multiplex immunofluorescence are employed to characterize tissue- and cell-specific expression patterns. Advances in single-cell RNA sequencing and digital spatial profiling have made it possible to map MMP expression within tissue microenvironments at single-cell resolution.
Genetic perturbation tools such as targeted gene editing and RNA interference are routinely used to elucidate isoform-specific functions in vitro and in vivo. Gain- and loss-of-function studies in 3D matrix models, organoids, and transgenic animals help to contextualize the roles of MMPs in cell migration, ECM remodeling, and tissue architecture.
Multiplex Profiling with Luminex xMAP Technology
One of the most transformative advancements in MMP analysis is the integration of Luminex xMAP technology, a bead-based multiplex assay platform capable of simultaneously quantifying multiple MMP isoforms from a single small-volume biological sample. Each microsphere in the xMAP system is internally dyed to create a unique spectral identity and externally conjugated with antibodies specific to individual MMPs. Upon sample incubation and reporter labeling, the platform quantitatively resolves the signal from each bead population via flow-based detection.
This approach offers several key advantages:
- Simultaneous quantification of multiple MMPs, including MMP-1, -2, -3, -7, -8, -9, -10, -12, and -13, in one assay, enabling integrative profiling of protease networks.
- High analytical sensitivity and dynamic range suitable for detecting low-abundance targets in plasma, tissue extracts, cerebrospinal fluid, or conditioned media.
- Scalability and reproducibility, which are essential for translational research, biomarker validation, and preclinical drug evaluation.
To support this application, Creative Proteomics offers a specialized Human MMP Panel Service based on Luminex xMAP technology. This platform is fully customizable and optimized for high-throughput studies, combining validated antibody pairs, robust assay protocols, and comprehensive data support. It is particularly well-suited for researchers in oncology, fibrosis, immunology, and chronic inflammation, where understanding the MMP landscape is crucial to modeling disease progression and therapeutic response.
Imaging and Systems-Level Integration
Fluorescence-based imaging, such as confocal and multiphoton microscopy, enables real-time visualization of MMP localization and matrix remodeling in live tissues and engineered matrices. MMP-responsive biosensors and genetically encoded reporters are increasingly used to monitor pericellular proteolysis in real time under defined mechanical and biochemical conditions.
At the systems level, proteomics and network-based computational modeling are employed to integrate MMP activity data with other omics layers—transcriptomics, phosphoproteomics, and metabolomics—enabling the reconstruction of signaling networks and regulatory circuits that drive pathological remodeling.
![]() For a deeper understanding of how to select, customize, and interpret MMP panels for complex research needs, refer to our detailed guide: Matrix Metalloproteinase Panels: Selection, Customization, and Best Practices.
For a deeper understanding of how to select, customize, and interpret MMP panels for complex research needs, refer to our detailed guide: Matrix Metalloproteinase Panels: Selection, Customization, and Best Practices.
References:
- Löffek, Stefanie, Oliver Schilling, and Claus-Werner Franzke. "Biological role of matrix metalloproteinases: a critical balance." European Respiratory Journal 38.1 (2011): 191-208.
- Schuppan, D., & Hahn, E. G. (2000). MMPs in the gut: inflammation hits the matrix. Gut, 47(1), 12-14.
- Cabral-Pacheco, G. A., Garza-Veloz, I., et al. (2020). The roles of matrix metalloproteinases and their inhibitors in human diseases. International journal of molecular sciences, 21(24), 9739.

