Introduction to Luminex xMAP
Luminex xMAP technology is a bead-based multiplexing platform that enables the simultaneous detection of multiple analytes (such as proteins, nucleic acids, or other biomarkers) in a single sample. This technology is widely used in diagnostics, research, and clinical testing due to its ability to provide fast, accurate, and high-throughput analysis.
At the heart of this system are color-coded magnetic or non-magnetic beads, each tagged with different capture agents. By using a range of these beads, researchers can test for several biomarkers in one reaction, offering unparalleled efficiency and data richness.
Principles of Luminex xMAP Technology
At its core, Luminex xMAP technology relies on color-coded microspheres (5.6 µm in diameter) embedded with precise ratios of infrared and red fluorescent dyes. Each bead set is assigned a unique "spectral fingerprint" (e.g., Bead Region 35 for IL-6 detection), enabling simultaneous identification of multiple targets.
Workflow breakdown
A. Bead functionalization: Antibodies or DNA probes are covalently coupled to bead surfaces.
B. Sample incubation: Analytes bind specifically to their target beads.
C. Detection: A secondary fluorescent reporter (e.g., phycoerythrin) quantifies bound molecules.
D. Data acquisition: Dual lasers in the xMAP instrument (e.g., MAGPIX) decode bead identity (red laser) and measure analyte concentration (green laser).
This bead-based multiplexing approach reduces sample volume requirements by 90% compared to ELISA, while maintaining sensitivities down to 0.1 pg/mL.
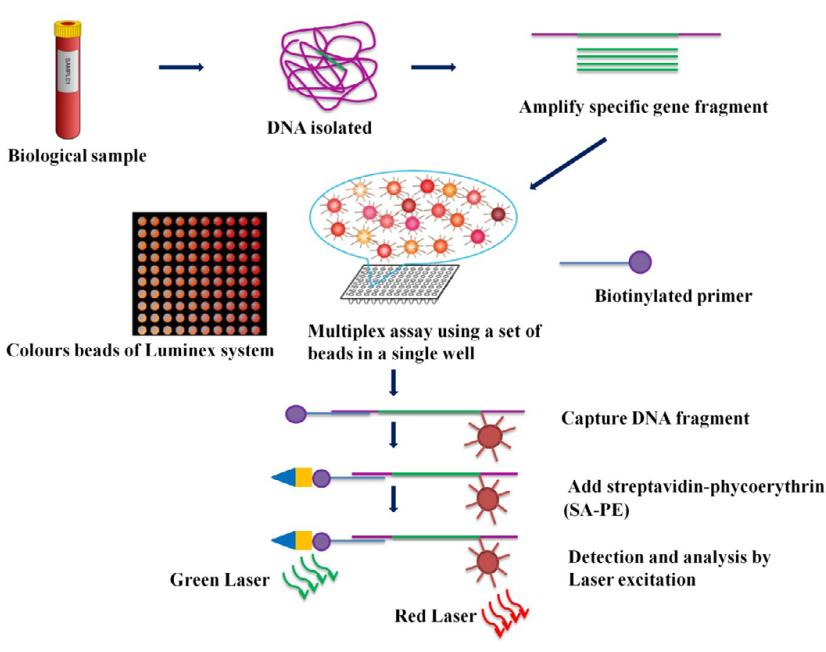 Principle of Luminex assay (Ranjan et al., 2015)
Principle of Luminex assay (Ranjan et al., 2015)
Services you may be interested in:
Components and Platforms of Luminex xMAP
Luminex xMAP technology is made up of various components that work together to deliver precise, multiplexed assay results. These components include color-coded beads, detection instruments, and the software that ties everything together. Luminex offers different systems and platforms to meet the diverse needs of research and diagnostic environments, ranging from smaller-scale research labs to large, high-throughput clinical diagnostics.
Luminex® 200™: The Pioneer of Flow Cytometry-Based Multiplexing
The Luminex® 200™ system is the original flagship platform for Luminex xMAP technology. As one of the earliest multiplex systems, it helped establish the commercial viability of bead-based assays, laying the groundwork for future advancements in multiplexed diagnostics and research. The Luminex® 200™ is ideal for smaller-scale projects and academic environments that require flexibility and ease of use.
- Core Technology: The system relies on flow cytometry principles, utilizing dual-laser technology—a red classification laser (635 nm) and a green detection laser (532 nm)—to both identify the spectral codes of the color-coded beads and detect the report molecules, such as PE fluorescence. This setup enables simultaneous detection of multiple analytes with high sensitivity.
- Detection Capability: The Luminex® 200™ supports up to 100-plex detection, meaning that up to 100 different biomarkers can be analyzed in a single well. The system is highly sensitive with a dynamic range of 3.5 logs, allowing for detection as low as 0.1 pg/mL.
- Applications: The Luminex® 200™ is suitable for small-scale research projects (such as single cytokine panels), teaching laboratories, and budget-conscious institutions. Its open bead programming capability (allowing for user-defined bead encoding) adds flexibility for researchers needing customizable assays.
- Speed: For a typical 96-well plate, it takes around 4 hours to complete an analysis (including manual wash steps).
- Cost: On the second-hand market, the Luminex® 200™ ranges from $15,000 to $25,000, making it an affordable choice for many research labs.
MAGPIX®: The Innovator in Magnetic Bead and Imaging Technology
The MAGPIX® system represents a significant innovation in Luminex's xMAP platform lineup. By integrating magnetic bead technology with CCD imaging, MAGPIX reduces the complexity of traditional flow-based systems, making it a valuable tool for clinical and mid-throughput applications.
- Imaging Upgrade: The MAGPIX® uses LED excitation sources (523 nm and 658 nm) combined with CCD imaging technology, which eliminates the need for the flow cytometry fluidics system. This results in reduced system maintenance and improved operational efficiency.
- Magnetic Bead Optimization: MAGPIX® employs MagPlex® magnetic beads, which are 6.5 μm in diameter and offer superior bead separation through magnetic isolation. This simplifies the washing process and reduces the time required for sample processing, enabling analysis to be completed in about 1.5 hours per 96-well plate.
- Cost-Effectiveness: MAGPIX® provides a lower-cost option compared to Luminex® 200™, with an initial purchase price approximately 30% less. It is ideal for clinical diagnostics (such as tumor marker screening) and mid-size research projects that need a balance between throughput and budget.
- Detection Capability: MAGPIX® supports up to 50-plex detection per sample. Its sensitivity is still impressive but may be limited for assays that require extremely high multiplexing.
- Speed: It is optimized for 1.5 hours per 96-well plate with automated wash steps.
- Cost: The MAGPIX® system costs between $35,000 and $45,000 for a new system, providing a cost-effective alternative for high-quality multiplexing at a reasonable price point.
Luminex® xMAP® INTELLIFLEX™: The Benchmark for High-Throughput Automation
The INTELLIFLEX™ system is Luminex's latest, most advanced platform, designed for high-throughput applications requiring enhanced sensitivity, automation, and flexibility. It represents the next generation of multiplexing with improved optics, faster processing, and increased data accuracy.
- Dynamic Fluid Control: A key innovation of the INTELLIFLEX™ system is its use of acoustic focusing technology to prevent bead aggregation, thereby ensuring stable and consistent results. This innovation helps reduce the coefficient of variation (CV), keeping it below 5% across different assays.
- Smart Expandability: The INTELLIFLEX™ platform is designed for extensive multiplexing capabilities, supporting 500-plex detection with potential future upgrades to 1,000-plex. The system is also highly compatible with third-party reagents, such as Bio-Rad's antibody-conjugated magnetic beads, making it adaptable for specialized assays.
- Applications: The INTELLIFLEX™ system is particularly suited for large-scale biomarker validation in drug development, as well as multi-omics integration. It is capable of processing over 10,000 samples per month, making it ideal for Contract Research Organizations (CROs) and large pharmaceutical labs.
- Speed: This system offers fast analysis, completing a 96-well plate in 30 minutes with fully automated loading and wash cycles, significantly reducing turnaround times in high-throughput environments.
- Cost: The INTELLIFLEX™ is the most expensive of the three platforms, with prices starting at $150,000+ for a new system. However, this investment is justified by its high-throughput capabilities, speed, and enhanced multiplexing power.
Performance Comparison of the Three Platforms
Here's a comparative look at the key specifications and capabilities of the three Luminex xMAP platforms:
| Parameter | Luminex® 200™ | MAGPIX® | INTELLIFLEX™ |
|---|---|---|---|
| Detection Throughput | 100-plex per well | 50-plex per well | 500-plex per well (theoretical max) |
| Speed | ~4 hours per 96-well plate (manual wash) | ~1.5 hours per 96-well plate (auto wash) | ~30 minutes per 96-well plate (auto load) |
| Suitable Applications | Research, Teaching, Small-scale studies | Clinical diagnostics, Mid-size research | Drug development, Multi-omics integration |
| Sensitivity | 0.1 pg/mL detection limit | Comparable to Luminex® 200™ | High sensitivity, up to 1,000-plex possible |
Luminex xMAP Beads and Their Role in Assays
The core advantage of Luminex xMAP technology lies in its unique bead encoding system and functional design. These beads are not only carriers for detection but also key tools for achieving high-throughput, multi-parameter analysis.
Structure and Encoding Mechanism of the Beads
- Materials and Size: xMAP beads are composed of 5.6 μm polystyrene, with surface activation using carboxyl groups to enable covalent coupling with antibodies or probes. The micron-scale size ensures both suspension stability and minimizes spatial hindrance effects found in solid-phase carriers (such as ELISA plates).
- Fluorescent Encoding: By adjusting the ratio of two infrared (658 nm) and red (635 nm) fluorescent dyes, beads can generate up to 500 distinct spectral profiles (earlier systems supported 100), each corresponding to a specific target. For instance, beads encoded with Region 25 are used for IL-6 detection, while Region 50 is for TNF-α detection.
- Magnetic vs. Non-Magnetic Beads:
- MagPlex® Magnetic Beads: Featuring an iron oxide core, these beads support magnetic separation, which reduces the washing steps from 5 to 1, significantly shortening assay time (e.g., MAGPIX system detection takes just 1.5 hours).
- Non-Magnetic Beads: These beads are suitable for traditional flow-based systems (e.g., Luminex® 200™), offering greater flexibility but requiring manual washing.
Functionalization and Detection Role of Beads
- Capture Molecule Coupling: Beads' surfaces are covalently linked with capture antibodies, antigens, or nucleic acid probes via EDC/NHS chemical crosslinking. For example, in COVID-19 antibody detection, Spike protein antigens are conjugated to specific beads to capture IgG antibodies in serum.
- Signal Generation Mechanisms:
- Sandwich Assay: For cytokine detection, capture antibodies on beads bind to the target protein, and then biotinylated detection antibodies and streptavidin-PE (SA-PE) generate a signal complex. The fluorescence intensity of PE (excited by a 532 nm laser) is proportional to the target's concentration.
- Direct Assay: In antibody detection, beads are directly conjugated with antigens, and the antibody titer is quantified using PE-labeled secondary antibodies (e.g., anti-human IgG from Jackson ImmunoResearch).
Core Role of Beads in Multiplex Detection
- Target Capacity Extension: A single well can simultaneously mix 100 encoded beads, allowing the detection of up to 100 biomarkers (e.g., 48-plex human cytokine panels), far surpassing the single-target limitations of traditional assays like ELISA.
- Dynamic Range and Sensitivity: The beads feature low-density capture molecules (about 10⁶ molecules per bead), which minimizes steric hindrance and boosts detection sensitivity to as low as 0.1 pg/mL, with a 3.5-log linear range.
- Sample Compatibility: Suitable for complex samples such as serum, plasma, and cerebrospinal fluid. For example, in fungal infection detection, β-D-glucan-conjugated beads can simultaneously identify 8 different fungal antigens, requiring only 50 μL of sample for analysis.
Bead Types and Adaptability to Detection Scenarios
| Parameter | MagPlex® Magnetic Beads | Traditional Non-Magnetic Beads |
|---|---|---|
| Washing Efficiency | Magnetic separation, reducing wash time by 80% | Relies on centrifugation/vacuum filtration |
| Throughput | Suitable for mid-high throughput (50-plex/well) | Suitable for small-scale research (100-plex) |
| Application | Clinical diagnostics (e.g., tumor marker screening) | Research exploration (e.g., novel target discovery) |
Innovative Application Examples
SARS-CoV-2 Multi-Antigen Detection: By mixing beads conjugated with Spike, Nucleocapsid, and RBD antigens, a single well can quantify IgG/IgM antibody subclasses, aiding in vaccine efficacy evaluation.
Single-Cell Secretomics: Coupling xMAP beads with microfluidic technology allows for the capture of multiple cytokines (e.g., IL-2, IFN-γ) secreted by single cells, achieving single-cell resolution in secretomics.
Glycosylation Analysis: Custom glycoprotein-conjugated beads (e.g., GlcNAc-modified beads) are used to detect anti-glycosylation antibodies in cancer patients' bodily fluids, helping predict therapeutic responses.
Applications of Luminex xMAP Technology
Cytokine Networks and Immune Regulation Mechanisms Research
xMAP technology provides a comprehensive view for immunology research through multiplex cytokine detection (e.g., 48-plex human cytokine panel):
Immune Response Monitoring: Simultaneously tracks key factors in Th1/Th2/Th17 pathways (e.g., IL-2, IFN-γ, IL-4, IL-17A), quantifying the balance between inflammatory responses and immune tolerance, revealing molecular mechanisms of autoimmune diseases like rheumatoid arthritis.
Cellular Communication Analysis: In tumor microenvironment studies, detecting chemokines (e.g., CXCL8, CCL2) and growth factors (e.g., VEGF, TGF-β) constructs cell signaling networks, elucidating the molecular basis of immune evasion.
Signaling Pathways and Protein Post-Translational Modification Research
Through customized microsphere conjugation strategies, xMAP technology deeply explores protein function regulation:
Phosphorylated Protein Quantification: In kinase-substrate pathway studies, simultaneously detecting phosphorylation markers (e.g., p-ERK, p-AKT) of MAPK and PI3K/AKT pathways reveals signaling dynamics under drug intervention.
Ubiquitination and Acetylation Analysis: Using specific modification antibodies, quantifying histone modifications (e.g., H3K27ac) or tumor suppressor proteins (e.g., p53) elucidates epigenetic regulation mechanisms.
Application Cases: In neurodegenerative disease models, detecting Tau protein phosphorylation sites (p-Tau181, p-Tau231) and Aβ42/Aβ40 ratios explores molecular pathological markers of Alzheimer's disease.
Single-Cell Secretomics and Cellular Heterogeneity Research
Combining microfluidics, xMAP technology drives single-cell resolution multi-omics research:
Single-Cell Cytokine Secretion Profiles: Capturing factors secreted by individual T cells (e.g., IL-2, TNF-α) reveals functional heterogeneity of immune cells, identifying rare subpopulations (e.g., tumor-specific T cells).
Cellular Metabolite Detection: In stem cell differentiation studies, synchronously detecting metabolic products (e.g., lactate, ATP) and differentiation markers (e.g., SOX2, OCT4) establishes associations between metabolic reprogramming and fate determination.
Pathogen-Host Interaction Mechanism Elucidation
xMAP technology provides high-throughput tools for microbiology and virology research:
Viral Invasion Mechanism Studies: Through multiplex detection of host receptors (e.g., ACE2, TMPRSS2) and inflammatory factors (e.g., IL-6, IL-1β), it reveals the immunopathological mechanisms of SARS-CoV-2 infection.
Bacterial Resistance Analysis: In antibiotic resistance studies, synchronously detecting resistance genes (e.g., mecA, blaCTX-M) and virulence factors (e.g., enterotoxin) tracks the evolutionary trajectory of resistant strains.
Genomics and Epigenomics Integrated Research
xMAP's nucleic acid detection capability (xTAG technology) supports multi-omics cross-research:
SNP Typing and Gene Expression Association: In GWAS studies, multiplex detection of SNP sites (e.g., rs12979860 with hepatitis C treatment response) and plasma miRNA levels uncovers genotype-phenotype associations.
DNA Methylation Spectrum Analysis: Customized microsphere-conjugated methylation-sensitive probes enable high-throughput screening of tumor suppressor gene (e.g., BRCA1, MGMT) promoter methylation in tumor samples.
Learn more
Metabolomics and Biomarker Discovery
xMAP technology drives precision medicine research through small molecule metabolite detection:
Lipid Metabolism Spectrum Analysis: In obesity models, synchronously detecting fatty acids (e.g., palmitic acid), adiponectin, and inflammatory factors elucidates the molecular network of metabolic syndrome.
Neurotransmitter Quantification: In Parkinson's disease research, jointly detecting dopamine, 5-HT, and their metabolites evaluates the biomarker combination for neurodegenerative changes.
Luminex xMAP vs Other Technologies
| Parameter | Luminex xMAP Technology | ELISA (Enzyme-Linked Immunosorbent Assay) | Luminex MAGPIX® | RT-PCR (Real-Time PCR) | Flow Cytometry |
|---|---|---|---|---|---|
| Detection Method | Bead-based multiplexing with fluorescence detection | Colorimetric or chemiluminescent detection | Magnetic bead-based multiplexing with CCD imaging | Quantification of nucleic acids via PCR amplification | Cell-based analysis with fluorescent tags |
| Multiplexing Capacity | Up to 500 targets per well (theoretical maximum) | Typically 1-2 targets per well | Up to 50-plex per well | Limited multiplexing (usually 1-5 targets per reaction) | Limited multiplexing (usually 5-20 markers per sample) |
| Sensitivity | 0.1 pg/mL (high sensitivity for low-abundance targets) | Moderate sensitivity (varies with kit quality) | High sensitivity, similar to Luminex xMAP | Very high sensitivity (detects small amounts of DNA/RNA) | High sensitivity, depending on fluorochromes used |
| Dynamic Range | 3.5 logs | Limited range (usually 2-3 logs) | Comparable to Luminex xMAP | Wide dynamic range (can detect low to high copy numbers) | Wide dynamic range (depends on specific assay) |
| Sample Volume | As low as 25-50 µL | Requires higher volumes (typically 50-100 µL) | As low as 25 µL | Requires 10-20 µL for PCR reactions | Requires 50-100 µL per sample |
| Throughput | Medium to high throughput (up to 96-well plates) | Low throughput (1-2 samples at a time) | Medium throughput (up to 96-well plates) | High throughput (can process multiple samples at once) | High throughput (can analyze hundreds of cells per second) |
| Automation | Fully automated or semi-automated options available | Typically manual or semi-automated (depends on kit) | Fully automated system (especially for MAGPIX) | Fully automated (PCR machines) | Fully automated (for flow cytometry-based analysis) |
| Cost per Test | Lower cost per sample compared to ELISA for multiplexing | Higher cost per sample due to single analyte focus | Similar to Luminex xMAP, but slightly more affordable | High cost per test (requires specific reagents and equipment) | Moderate to high cost (depending on reagent and equipment) |
| Flexibility | Highly customizable (user-defined bead encoding) | Limited flexibility (predefined kits and assays) | Customizable with available beads and assays | Limited to specific target sequences (e.g., gene primers) | High flexibility, but more focused on cell-based assays |
| Application Areas | Broad: Proteomics, diagnostics, multi-omics, biomarkers | Immunology, diagnostics, cytokine analysis | Clinical diagnostics, tumor marker detection | Pathogen detection, genetic research, diagnostics | Cell analysis, immune profiling, surface marker analysis |
| Speed | Fast (typically 1-2 hours per 96-well plate) | Slow (usually 4-6 hours for a full plate) | Fast (1-2 hours per 96-well plate) | Fast (within hours for results, depending on assay) | Fast (depends on analysis complexity and markers) |
Luminex xMAP Protocols and Workflow (Luminex® 200™ System)
1. Pre-Experiment Preparation
Reagents and Consumables
Microsphere Selection: Only non-magnetic beads are supported, such as Luminex® xMAP® Microspheres, which require manual washing. It is recommended to use pre-set panels for efficiency.
Key Reagents:
- Sheath Fluid (xMAP Sheath Fluid, Catalog Number: 40-50000)
- PE-labeled Detection Antibodies: Recommend using R&D Systems or BioLegend brands.
Sample Processing
Serum/Plasma: Avoid using heparin as an anticoagulant (may interfere with microsphere suspension), recommend EDTA or sodium citrate.
Tissue Lysis Buffer: Suggest controlling total protein concentration between 1-5 mg/mL to avoid high lipid interference in fluidics.
Instrument Calibration
Laser Calibration: Perform laser preheating daily after startup (red classification laser 635 nm, green detection laser 532 nm), with power stabilized at 12.5 mW (red) and 10 mW (green).
Fluidics Detection: Confirm sheath fluid flow rate at 60 μL/min through the Prime step, with flow stability (CV <2%) being crucial for data quality1.
2. Experimental Procedure (Using Sandwich Assay as an Example)
Microsphere Activation and Conjugation (Required for Custom Detection)
Activation: Take 5×10⁶ carboxylated microspheres, add 50 μL of 100 mM MES buffer (pH 6.0), 10 μL freshly prepared EDC (50 mg/mL), and 10 μL NHS (50 mg/mL), shake at room temperature for 20 minutes.
Conjugation: Add 50 μg capture antibody (dissolved in pH 7.4 PBS), shake at room temperature in the dark for 2 hours, block with Blocking Buffer (1% BSA) for 30 minutes.
Detection Steps
| Step | Conditions | Key Parameters |
|---|---|---|
| Microsphere Equilibration | Ultrasonic treatment (40 kHz) for 1 minute + vortex for 30 seconds | Final microsphere concentration: 1,200/μL/Region |
| Sample Incubation | 50 μL sample + 50 μL microspheres, shake at 750 rpm for 2 hours | Avoid air bubbles interfering with fluidics |
| Washing (Non-Magnetic) | Vacuum filtration (Millipore MultiScreen® plate) or centrifugation (500×g, 5 minutes) | Wash three times, each with 200 μL PBS-T |
| Detection Antibody Incubation | Biotinylated antibody (2 μg/mL), room temperature for 1 hour | Perform in the dark, shake at ≤600 rpm |
| Signal Amplification | SA-PE (8 μg/mL), room temperature for 30 minutes | PE concentration needs optimization based on pre-experiments |
Machine Detection
Luminex 200™ Settings:
- Acquisition Time: 60 seconds/well (ensure ≥100 microspheres are collected per Region).
- Signal Gain: Suggest setting green laser PMT voltage to 650-750 V to avoid signal saturation.
- Exclusion Threshold: Set microsphere diameter range to 4.5-6.5 μm to exclude debris interference.
3. Data Analysis and Quality Control
Data Acquisition Software
xPONENT® 3.1: Automatically calculates MFI values, supports dynamic range compression (DRC) to prevent signal overflow from high-concentration samples.
Quality Control Standards
- Microsphere Distribution: CV of microsphere count per Region<15%, CV of diameter distribution <8%.
- Standard Curve: Use 8-point dilution standards (R² ≥0.99), LLOQ (lower limit of quantification) should reach 0.5 pg/mL (e.g., IL-6 detection).
- Intra- and Inter-Plate Variability: Require intra-plate CV <10%, inter-plate CV <15%.
Innovation in Luminex xMAP Technology
The Intelliflex system represents the next generation of Luminex xMAP technology, featuring significant advancements in optics and data quality. The system uses an upgraded optical platform to enhance the sensitivity and resolution of assays, allowing for more accurate and detailed multiplexing results.
One of the key innovations in Intelliflex is the ability to measure multiple colors of fluorescence simultaneously, enabling even higher levels of multiplexing. This makes the system particularly suited for complex research projects and diagnostic tests that require detection of a wide range of targets.
The continuous innovation in xMAP technology shows great promise for the future, with improvements in assay sensitivity, automation, and data analysis on the horizon.
Luminex Multiplex Assay in Creative Proteomics
Creative Proteomics offers comprehensive Luminex multiplex detection services, covering both pre-set standardized detection panels and customized development services. These services provide efficient solutions for fields such as immunology, oncology, and metabolic diseases. Below are examples of popular Luminex Cytokine Panels:
- Detection Indicators: TNF-α, IL-1α, IL-17A, and other cytokines.
- Application Scenarios: Analyzing inflammatory signals from cancer-associated fibroblasts (CAFs) in tumor microenvironments, such as studying the resistance mechanisms of neoadjuvant therapy in colorectal cancer.
- Detection Indicators: GM-CSF, IL-2, IL-10, and other key factors.
- Application Scenarios: Evaluating immune responses in vaccine development, such as analyzing the TH1/TH17 cytokine spectrum for IDH1 mutant glioma vaccines.
Whether you are exploring basic mechanisms or accelerating clinical translation, Creative Proteomics' Luminex technology can empower your research. Contact us to learn more.
Reference:
- Ranjan, Koushlesh, P. Minakshi, and Gaya Prasad. "Application of molecular and serological diagnostics in veterinary parasitology." J Adv Parasitol 2.4 (2015): 80-99.

