Matrix metalloproteinases (MMPs) are a structurally related family of zinc-dependent endopeptidases responsible for the degradation and remodeling of extracellular matrix (ECM) components. Their activity plays a central role in fundamental biological processes such as tissue development, repair, angiogenesis, and immune regulation. Due to their tightly controlled but highly responsive expression patterns, MMPs are frequently studied as indicators of microenvironmental change in models of inflammation, fibrosis, and tissue remodeling.
In experimental systems, changes in MMP expression or activity often serve as molecular readouts for ECM dynamics, protease-inhibitor balance, and cellular responses to environmental cues. Whether analyzing stromal remodeling in 3D cultures or matrix turnover in animal tissue lysates, obtaining meaningful MMP data requires both methodological precision and strategic panel selection.
![]() For an in-depth overview of MMP classification, structure, and biological roles, see Matrix Metalloproteinases (MMPs): Function, Biology, and Research Applications.
For an in-depth overview of MMP classification, structure, and biological roles, see Matrix Metalloproteinases (MMPs): Function, Biology, and Research Applications.
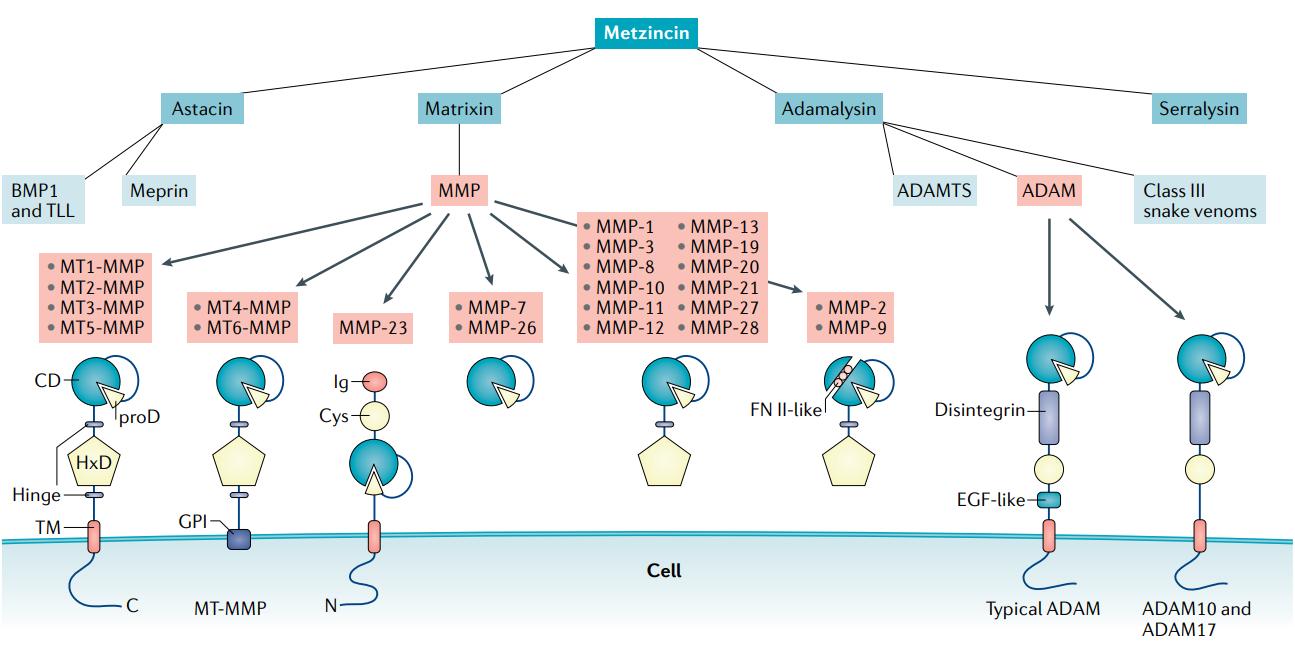 The metzincin family of zinc proteases (Wozniak, Justyna, et al., 2021).
The metzincin family of zinc proteases (Wozniak, Justyna, et al., 2021).
Why Panel Design Matters in MMP Research
MMPs exhibit significant isoform diversity, overlapping substrate specificities, and complex regulation at the level of activation and inhibition. These characteristics create analytical challenges that cannot be resolved by generic assays or one-size-fits-all panels. Instead, the relevance and reproducibility of MMP data depend on thoughtful alignment between:
- The specific isoforms selected and their biological relevance to the study system,
- The assay platform used, including its sensitivity, specificity, and multiplexing capabilities,
- The form of MMP detected (latent vs active),
- The sample type and matrix composition, which can profoundly affect assay performance.
What This Guide Covers
This article provides a structured overview to support researchers in selecting or customizing MMP panels for robust and interpretable results. Topics include:
- Comparative analysis of MMP panel formats and technologies
- Guidance on tailoring isoform selection to biological questions
- Sample-specific considerations and matrix interference mitigation
- Identification of common pitfalls and strategies for assay validation
- Practical approaches to data analysis and biological interpretation
By addressing both technical and experimental design dimensions, this guide aims to help researchers enhance the reliability and relevance of MMP measurements in complex biological systems.
Types of MMP Panels
Selecting the right type of MMP panel begins with understanding how assay design influences biological insight. Different panel formats offer trade-offs in sensitivity, throughput, and contextual resolution.
Panel Type Comparison
| Panel Type | Description | Suitable For |
|---|---|---|
| Singleplex | One target per assay; typically ELISA-based | Focused mechanistic studies, validation of single targets |
| Multiplex | Multiple targets in one well/sample; often bead- or array-based | Exploratory profiling, limited sample volumes, systems-level insight |
| Customizable | Pre-designed or fully custom selections, including emerging isoforms | Niche research needs, lesser-studied MMPs, method development |
Each panel type should be evaluated not only by the number of analytes but by assay performance characteristics such as detection range, cross-reactivity control, and compatibility with intended sample matrices.
Common Analytical Platforms
Choosing the right analytical platform is central to successful MMP profiling. Each technology varies in terms of sensitivity, throughput, isoform resolution, and compatibility with sample matrices. Among these, Luminex xMAP has emerged as the preferred choice for multi-target MMP analysis in complex biological studies.
ELISA (Enzyme-Linked Immunosorbent Assay)
- High specificity and quantifiability
- Single-analyte format; each MMP requires a separate well
- Limited scalability for high-throughput or systems-level studies
Luminex xMAP Technology – Preferred for Multiplexed MMP Profiling
Luminex xMAP is a bead-based platform that combines the throughput of flow cytometry with the precision of immunoassays. Each microsphere is internally dyed and conjugated with a capture antibody specific to a given MMP, allowing simultaneous detection of up to 50 analytes in a single well.
Key Advantages:
✓ High Multiplexing Capacity: Profile broad MMP panels—including MMP-1, -2, -3, -7, -9, -10, -12, -13, and TIMPs—simultaneously using just 25–50 μL of sample.
✓ Matrix Flexibility: Validated across serum, plasma, tissue lysates, BALF, and culture media.
✓ Quantitative Accuracy: Strong correlation with ELISA data; better dynamic range.
✓ Efficient Sample Usage: Essential for precious samples such as clinical plasma or primary cell supernatants.
![]() Creative Proteomics offers a fully optimized Human MMP Panel Service, powered by Luminex xMAP technology. The service enables high-throughput, low-volume quantification of multiple MMPs and their inhibitors with exceptional reproducibility, making it ideal for inflammation, cancer, fibrosis, and ECM remodeling research.
Creative Proteomics offers a fully optimized Human MMP Panel Service, powered by Luminex xMAP technology. The service enables high-throughput, low-volume quantification of multiple MMPs and their inhibitors with exceptional reproducibility, making it ideal for inflammation, cancer, fibrosis, and ECM remodeling research.
Western Blot & Zymography
- Semi-quantitative and labor-intensive
- Useful for distinguishing pro-enzyme vs active forms
- Low throughput and less scalable for screening applications
Enzymatic Activity Assays
- Directly measure proteolytic function using fluorogenic substrates
- Reflects net MMP activity, integrating effects of activation and inhibition
- Susceptible to interference from endogenous TIMPs or matrix components
Key MMP Targets in Research
Matrix metalloproteinases comprise over 20 structurally related but functionally distinct isoforms. A well-designed MMP panel should align with the biological process under investigation by including isoforms relevant to tissue-specific remodeling, inflammation, or degradation of ECM components.
Most Commonly Profiled MMP Isoforms
| MMP Isoform | Subfamily | Substrate Preference | Key Research Contexts |
|---|---|---|---|
| MMP-1 | Collagenase | Interstitial collagens (I, II, III) | ECM remodeling, fibrosis models |
| MMP-2 | Gelatinase | Gelatin, collagen IV, elastin | Angiogenesis, basement membrane degradation |
| MMP-3 | Stromelysin | Proteoglycans, laminin, fibronectin | Inflammation, joint models |
| MMP-7 | Matrilysin | ECM and non-ECM proteins | Epithelial remodeling, innate immunity |
| MMP-9 | Gelatinase | Gelatin, elastin, collagen IV | Leukocyte infiltration, barrier disruption |
| MMP-12 | Metalloelastase | Elastin, fibronectin | Macrophage-driven tissue damage |
| MMP-13 | Collagenase-3 | Type II collagen | Cartilage degradation, fibrosis |
| MMP-14 | Membrane-type | Pro-MMP-2 activation, cell surface ECM | Pericellular remodeling, invasion studies |
Subfamily Overview and Mechanistic Roles
Collagenases (MMP-1, -8, -13)
- Break down triple-helical interstitial collagens, initiating matrix degradation.
- Central in fibrosis, wound healing, and cartilage breakdown studies.
Gelatinases (MMP-2, -9)
- Degrade denatured collagen and basement membrane proteins.
- Frequently upregulated in models of inflammation, vascular remodeling, and cell migration.
Stromelysins (MMP-3, -10, -11)
- Broad substrate range including non-collagen ECM components.
- Often co-expressed with other MMPs and facilitate their activation.
Membrane-Type MMPs (MT-MMPs; e.g., MMP-14)
- Anchor to the cell membrane and regulate pericellular proteolysis.
- Play a role in cell invasion, angiogenesis, and activation of pro-MMPs.
Panel Composition by Research Focus
To ensure data relevance, MMP panel design should be tailored to the biological pathway or disease model:
- Cancer Research: MMP-2, -9, -14 (tumor invasion, basement membrane degradation)
- Fibrosis and ECM Remodeling: MMP-1, -13, -3 (collagen breakdown, fibroblast activity)
- Inflammatory Models: MMP-7, -9, -12 (macrophage activity, immune cell recruitment)
- Tissue Regeneration & Repair: MMP-2, -3, -10 (matrix turnover, epithelial-stromal interactions)
Customizing MMP Panels According to Research Needs
Off-the-shelf MMP panels often serve as convenient starting points, but they rarely offer the biological precision required for hypothesis-driven research. Customization—whether through selection of isoforms, adjustment of assay format, or adaptation to specific matrices—enables a more targeted, interpretable, and scientifically robust approach.
Biological Relevance of Isoforms
The first step is to align panel content with your experimental question and pathophysiological context. For instance:
- Studies on cartilage breakdown may prioritize MMP-1, -13, and -3.
- Epithelial barrier models may focus on MMP-7 and -9.
- Macrophage-mediated inflammation may include MMP-12, often overlooked in standard kits.
Inclusion of Pro-Enzyme and Active Forms
MMPs are synthesized as latent zymogens (pro-MMPs) that require activation to become enzymatically functional.
- Immunoassays (e.g., ELISA, Luminex) usually detect total protein levels—both active and inactive.
- Enzymatic activity assays or zymography can differentiate these forms, crucial for understanding functional proteolytic capacity.
- Including both formats provides a dual readout of expression and activity.
Tip: In systems where activation is stimulus-dependent (e.g., post-injury, cytokine stimulation), measuring only total MMP may obscure key regulatory mechanisms.
Membrane-Bound vs Secreted MMPs
Membrane-type MMPs such as MMP-14 (MT1-MMP) are often missed in soluble-target panels but play critical roles in pericellular remodeling and pro-MMP activation.
- Membrane MMPs require cell lysates or surface protein isolation.
- They may also be relevant in co-culture or invasion models where cell–matrix contact drives phenotype.
Fine-Tuning for Sample Type Compatibility
Different biological matrices exhibit vastly different MMP profiles—and different assay limitations:
| Sample Type | Typical Targets | Considerations |
|---|---|---|
| Plasma/Serum | MMP-2, -9 | Prone to platelet release artifacts (e.g., MMP-9); requires inhibitor use |
| Tissue Lysate | MMP-1, -3, -13 | High MMP content; protein degradation risk; variable matrix complexity |
| Cell Supernatant | MMP-2, -7, -9 | Useful for secretion dynamics; may require concentration |
| BALF/CSF/Urine | MMP-2, -9 | Low-abundance targets; high variability; matrix dilution effects |
Additional Matrix-Specific Customizations
- Matrix depletion or buffer exchange (e.g., via spin columns) can reduce interference.
- Protease inhibitor cocktails should be validated to avoid inhibition of active MMPs.
Case Example – Fibrosis Model
In a mouse liver fibrosis model, researchers may choose:
- MMP-2, -9: for early ECM degradation,
- MMP-13: for collagen resorption,
- MMP-14: for activation profiling,
- With zymography to monitor the MMP-2 activation cascade over time.
Summary Checklist for Panel Customization
- Define the primary biological hypothesis
- Identify MMP isoforms most relevant to your model
- Decide whether you need to distinguish active vs total MMP
- Match the panel to your sample type and volume constraints
- Consider adding orthogonal methods for validation
Sample Types and Matrix Considerations
The accuracy and reproducibility of MMP quantification are tightly linked to the sample matrix. Biological fluids, tissue lysates, and culture media each present unique biochemical environments that can enhance or obscure signal detection. Failure to account for matrix-specific interferences often leads to inconsistent or non-biologically meaningful results—even when using validated assay kits.
Matrix-Specific Challenges and Solutions
| Sample Type | Matrix Challenges | Key Mitigation Strategies |
|---|---|---|
| Plasma | Platelet-derived MMP-9 release during collection; anticoagulant interference | Use citrate/EDTA tubes; minimize freeze–thaw; centrifuge immediately |
| Serum | Artificial elevation of MMPs during clotting (esp. MMP-9, MMP-8) | Prefer plasma when targeting inflammatory MMPs |
| Tissue Lysate | High protease background; viscosity; inconsistent homogenization | Use protease inhibitors; standardize protein input; filter or clarify |
| Conditioned Media | Dilute MMP levels; accumulation over time may skew kinetics | Concentrate samples; normalize to cell count or timepoint |
| Urine, BALF, CSF | Low protein content; pH or salt variability; high inter-individual variation | Pre-concentration; buffer normalization; include matrix blanks |
Matrix Effects: Sources and Impact
Endogenous Inhibitors (e.g., TIMPs)
TIMPs (tissue inhibitors of metalloproteinases) are natural MMP antagonists. Their presence in fluids like serum or BALF can inhibit enzymatic assays, producing a false low reading of active MMPs despite high total protein.
Non-Specific Binding
Plasma proteins like albumin and globulins may non-specifically bind detection antibodies or MMPs themselves, causing signal suppression in immunoassays.
pH and Salt Effects
Some MMP assays are sensitive to pH or ionic strength, especially in urine or cerebrospinal fluid. Variability in these parameters can affect enzyme conformation or antibody-antigen interactions.
Interfering Proteins or Lipids
Hemolyzed samples or necrotic tissue lysates may contain high levels of hemoglobin, myoglobin, or lipids—potentially quenching fluorescent signals or interfering with colorimetric readouts.
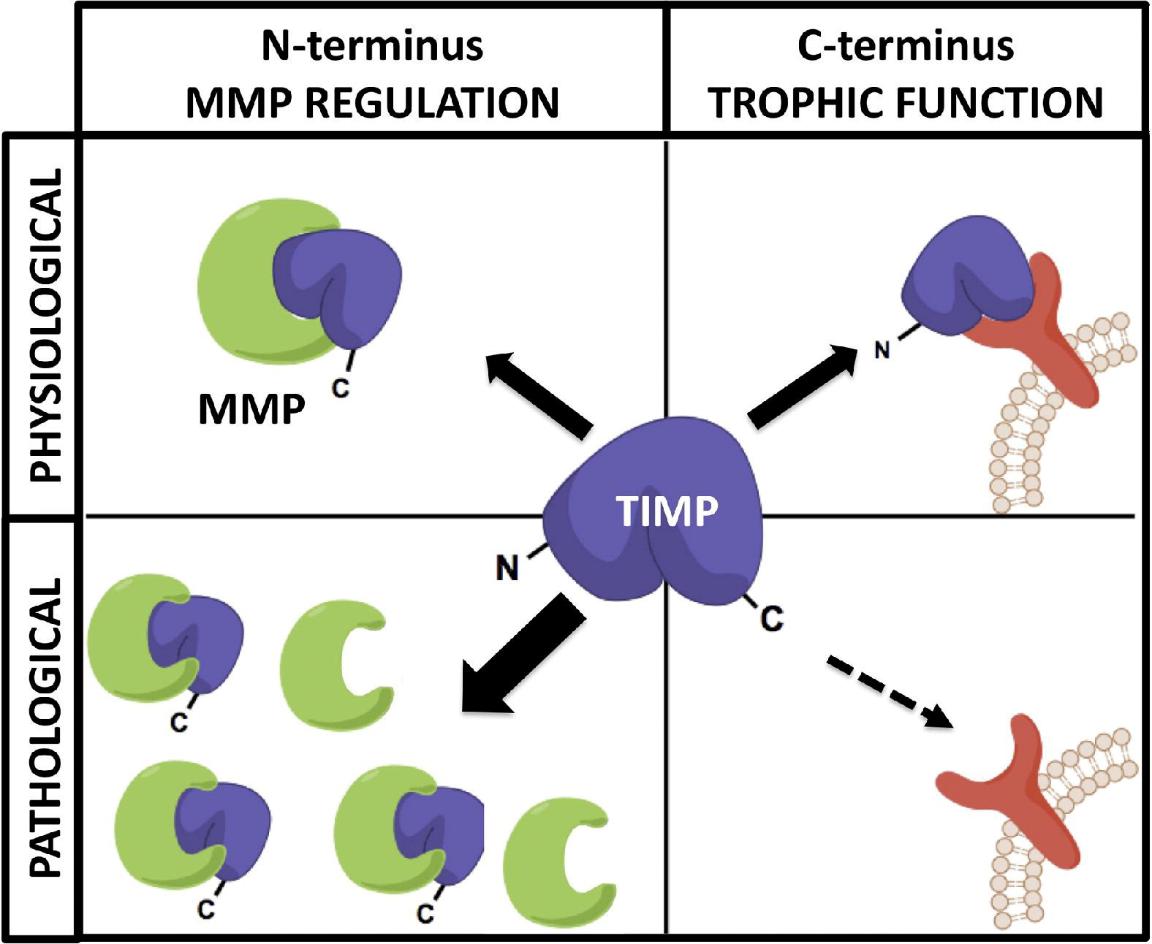 MMP-TIMP regulatory balance and signaling. Under normal conditions, TIMPs inhibit MMPs and also activate signaling via receptor binding. In disease states, elevated MMP levels lead to excessive proteolysis and reduced TIMP-mediated signaling (Moore et al., 2012).
MMP-TIMP regulatory balance and signaling. Under normal conditions, TIMPs inhibit MMPs and also activate signaling via receptor binding. In disease states, elevated MMP levels lead to excessive proteolysis and reduced TIMP-mediated signaling (Moore et al., 2012).
Best Practices to Reduce Matrix-Driven Artifacts
✓ Standardization
- Use the same sample type and processing method across experimental groups.
- Document and control pre-analytical variables (e.g., centrifugation speed, storage temperature, number of freeze–thaws).
✓ Spiking and Recovery
Add known quantities of recombinant MMPs to matrix-matched samples to assess recovery efficiency. Low recovery suggests inhibition or matrix binding.
✓ Dilution Linearity Tests
Perform serial dilutions to verify signal proportionality. Non-linear curves indicate interference or saturation.
✓ Buffer Optimization
Use matrix-compatible assay diluents (provided in multiplex kits or prepared with protein stabilizers and detergents) to reduce background noise.
Common Pitfalls and How to Avoid Them in MMP Panel Design and Analysis
Antibody Cross-Reactivity Undermines Specificity
Problem
Many MMP isoforms, especially those within the same subfamily (e.g., MMP-2 and MMP-9), share conserved catalytic domains. Commercial antibodies may lack isoform-level specificity, resulting in false-positive detection or inflated signals in multiplex panels.
Impact
- Loss of target specificity
- Misinterpretation of isoform-specific roles
- Poor reproducibility across platforms
Solutions
✓ Use antibodies validated with recombinant proteins and isoform-specific blocking experiments
✓ Confirm key analytes with orthogonal techniques (e.g., Western blot, LC-MS)
✓ Avoid panels lacking clear disclosure of cross-reactivity performance
Matrix Interference Compromises Quantification
Problem
Biological samples such as plasma, BALF, or tissue lysates contain high concentrations of interfering proteins, lipids, salts, and endogenous inhibitors. These components affect binding kinetics or quench assay signals.
Impact
- Nonlinear calibration curves
- Poor recovery rates
- Over- or underestimation of true analyte levels
Solutions
✓ Conduct spike-and-recovery tests to evaluate matrix-specific signal loss
✓ Perform serial dilutions to test linearity and identify optimal dilution factors
✓ Use matrix-matched standard curves when switching sample types
✓ Choose platforms like Luminex xMAP, which tolerate more matrix complexity than ELISA
Failure to Distinguish Total vs. Active MMPs
Problem
Standard immunoassays detect total protein levels (pro- and active MMPs combined), whereas only the active form contributes to matrix degradation. Enzyme-inhibitor complexes (e.g., MMP-TIMP) may still be measured despite being functionally silent.
Impact
- Overestimation of biological activity
- Missed activation events
- Discrepancy with phenotype
Solutions
✓ Integrate activity assays (e.g., FRET-based substrates) to quantify functional MMPs
✓ Use zymography for gelatinase activity visualization
✓ Measure TIMP levels in parallel to interpret inhibitory context
Improper Sample Handling Leads to Artifacts
Problem
MMPs are highly sensitive to post-collection degradation and ex vivo activation. Platelet activation in serum, proteolytic cleavage in lysates, and repeated freeze–thaw cycles can all alter analyte levels.
Impact
- Artificial elevation of MMP-9 from platelets
- Proteolytic loss of sensitive isoforms
- Inter-batch variability
Solutions
✓ Prefer plasma over serum for inflammation-related MMPs
✓ Add protease inhibitors immediately after sample harvest
✓ Minimize freeze–thaw cycles; use pre-aliquoted vials
✓ Standardize storage at –80°C with full metadata tracking
Detection Range Mismatch
Problem
Some biologically relevant MMPs are expressed at extremely low levels in certain matrices (e.g., MMP-12 in plasma), falling below assay LOD.
Impact
- False negatives
- Data misinterpretation due to assay limitations rather than biological absence
Solutions
✓ Concentrate dilute matrices (e.g., urine, CSF) before analysis
✓ Choose ultrasensitive platforms (e.g., Luminex, Meso Scale Discovery)
✓ Cross-check expected expression levels from transcriptomic/proteomic databases
Oversimplified Experimental Design
Problem
One-time-point sampling, lack of biological replicates, or omission of key controls reduces the interpretability and reproducibility of MMP data.
Impact
- Failure to capture temporal dynamics
- Spurious statistical significance
- Inability to replicate findings
Solutions
✓ Use time-course sampling to monitor dynamic expression changes
✓ Include biological replicates (≥3) and matched controls
✓ Record and normalize against variables such as total protein, cell number, or volume
Weak Data Validation and Interpretation
Problem
Complex datasets from multiplex panels are often interpreted based solely on p-values or fold changes, without biological integration.
Impact
- Overemphasis on statistically significant but biologically irrelevant changes
- Missed insight due to lack of systems-level interpretation
Solutions
✓ Normalize data using appropriate baselines (e.g., total protein, internal standards)
✓ Apply multivariate statistics (e.g., PCA, clustering) to uncover patterns
✓ Correlate MMP profiles with related markers (e.g., cytokines, ECM fragments)
Practical Summary Table
| Pitfall | Consequence | Solution |
|---|---|---|
| Antibody cross-reactivity | False positives | Validate with recombinant controls |
| Matrix interference | Signal suppression/enhancement | Spike/recovery & dilution linearity |
| Total vs active MMP confusion | Functional misinterpretation | Add activity assays or zymography |
| Sample handling inconsistency | Degraded or artifactual MMP levels | Standardized collection & storage |
| Low-abundance detection failure | False negatives | Use ultrasensitive methods or concentrate |
| Oversimplified design | Data not representative of biology | Include time points and replicates |
| Poor data interpretation | Misleading conclusions | Use bioinformatics and pathway integration |
Data Analysis and Interpretation
Multiplexed MMP assays generate rich datasets that require careful statistical handling and biological contextualization. Missteps in normalization, control application, or interpretation can easily obscure meaningful patterns.
Data Preprocessing and Normalization
Background Subtraction: Remove non-specific signal using blanks or negative controls.
Normalization: Adjust raw concentrations against:
- Total protein (for lysates)
- Cell number (for media)
- Volume or creatinine (for urine)
Inter-plate Calibration: Use internal standards across plates to correct for technical variation.
Statistical Approaches
Univariate Tests: Use ANOVA or non-parametric tests (e.g., Kruskal–Wallis) for single-MMP comparisons.
Multivariate Methods:
- Principal Component Analysis (PCA): Explore overall MMP profile patterns.
- Hierarchical Clustering: Identify co-expressed MMP groups or sample clusters.
Tip: Apply multiple testing correction (e.g., FDR) to control false positives when analyzing large panels.
Biological Interpretation
Cross-reference with Biological Function: Relate expression shifts to known MMP functions (e.g., MMP-9 and immune infiltration).
Balance with TIMPs: Consider the ratio of MMPs to TIMPs to infer net proteolytic potential.
Integrative Analysis: Combine MMP data with cytokines, ECM markers, or gene expression to build mechanistic narratives.
Final Thought
Quantitative data alone does not yield biological insight—interpretation depends on integration, context, and validation
References:
- Wozniak, Justyna, et al. "Key metalloproteinase-mediated pathways in the kidney." Nature Reviews Nephrology 17.8 (2021): 513-527.
- Moore, Craig S., and Stephen J. Crocker. "An alternate perspective on the roles of TIMPs and MMPs in pathology." The American journal of pathology 180.1 (2012): 12-16.

