Cytokines in Cell Culture
Cytokines are essential in regulating cell behavior in culture, influencing proliferation, differentiation, and survival. In immune cell cultures, cytokines like IL-2 and IFN-γ drive T cell activation, while growth factors such as EGF and FGF are key for stem cell maintenance. Selecting the right cytokines for cell culture ensures accurate modeling of physiological conditions and experimental consistency.
In disease models and co-culture systems, cytokines mediate intercellular communication, reflecting in vivo processes. Luminex cytokine assays and Luminex xMAP technology enable multiplex analysis, allowing simultaneous measurement of multiple cytokines in a single sample. This approach provides deeper insights into immune responses, tumor environments, and regenerative processes, supporting the identification of therapeutic targets.
Additionally, cytokine profiling plays a critical role in drug development. Changes in cytokine levels can signal immune activation or toxicity, making cytokine panels valuable tools in screening assays. By integrating multiplex cytokine assays, researchers can improve accuracy and reproducibility, accelerating progress in biomedical research.
Cytokine Selection and Application in Cell Culture
Cytokine Selection Based on Cell Type
The choice of cytokines depends primarily on the type of cells being cultured and their specific needs.
- Immune Cells: For T cell activation, cytokines like IL-2 are essential, while IFN-γ is commonly used to stimulate macrophages or dendritic cells.
- Stem Cells: For maintaining pluripotency in stem cell cultures, cytokines such as LIF (Leukemia Inhibitory Factor) are critical. Differentiation into specific lineages often requires cytokines like TGF-β, which induces mesodermal or ectodermal differentiation.
Cytokine Selection Based on Experimental Context
The experimental goals also influence cytokine choice. In disease models, cytokines are used to simulate physiological or pathological conditions.
- Tumor Microenvironment Models: Cytokines such as IL-6, TNF-α, and VEGF are often added to simulate the inflammatory environment of cancer, helping to model tumor progression, immune evasion, and angiogenesis in vitro.
- Immune Response Studies: When studying immune modulation, IL-4 and IFN-γ are essential for T cell polarization and macrophage activation. These cytokines help to mimic the immune system's response to infections or autoimmune diseases.
The selection of cytokines in these models should align with the specific signaling pathways being studied.
Cytokine Gradients for Studying Cellular Behavior
Creating cytokine gradients within cell culture systems is another powerful approach, particularly when studying chemotaxis, cell migration, or tissue development.
- Chemotaxis Studies: Gradients of cytokines like IL-8 or CXCL12 can be established to guide the migration of immune cells or stem cells, mimicking natural cellular migration processes during wound healing or immune response.
- Cancer Metastasis Models: By setting up cytokine gradients, researchers can investigate how cancer cells respond to migratory cues, aiding in the study of metastasis and the role of the tumor microenvironment.
These gradient-based models, often used in 3D culture systems, allow for more physiologically relevant studies that are difficult to replicate in traditional 2D cultures.
Optimizing Cytokine Combinations for Specific Outcomes
Beyond individual cytokine selection, combining multiple cytokines is often necessary to mimic more complex biological processes.
- Differentiation Protocols: For stem cells or progenitor cells, specific cytokine cocktails can guide differentiation into various lineages. For instance, the combination of TGF-β with other growth factors can promote mesodermal differentiation, while specific interleukins support hematopoietic lineage development.
- Tissue Regeneration and Repair: When studying tissue engineering or regenerative medicine, cytokines are chosen to promote cellular proliferation, migration, and matrix production. Cytokines such as FGF and EGF are commonly used in these applications to encourage wound healing or tissue regeneration.
Carefully designing cytokine combinations allows researchers to replicate specific in vivo processes, providing insights that are directly applicable to therapeutic development.
Methods for Cytokine Analysis in Cell Culture
Singleplex Cytokine Assays
Traditional methods for cytokine analysis include singleplex assays, which measure one cytokine at a time.
- Enzyme-Linked Immunosorbent Assay (ELISA): ELISA is the gold standard for measuring cytokine concentrations. It offers high sensitivity and specificity, which makes it ideal for detecting low-abundance cytokines. ELISA involves a solid-phase immunoassay where the cytokine binds to specific antibodies on a plate, followed by detection with an enzyme-conjugated secondary antibody. Although ELISA provides accurate and reliable results, it is limited in throughput, requiring a separate assay for each cytokine of interest.
- Western Blotting: Another method for cytokine analysis is Western blotting, which detects specific proteins using antibodies. While it can confirm the presence of cytokines, it does not provide quantitative data as accurately as ELISA and is not well-suited for multiplex analysis.
Multiplex Cytokine Assays
As cytokine networks are complex and often involve the interaction of multiple cytokines, multiplex assays provide a more comprehensive approach to cytokine analysis. Multiplex assays allow the simultaneous detection of multiple cytokines in a single sample, saving time and sample volume.
- Luminex xMAP Technology: The Luminex xMAP platform is one of the most widely used technologies for multiplex cytokine analysis. This bead-based system uses color-coded beads, each conjugated with a specific capture antibody. The beads are mixed with the sample, and cytokines bind to the capture antibodies. The cytokine levels are quantified by detecting the specific bead type and measuring the intensity of the fluorescent signal using a flow-based system. Luminex assays allow the simultaneous detection of up to 100 different cytokines in a single sample.
- Sensitivity and Specificity: One of the advantages of Luminex cytokine assays is their ability to detect low-abundance cytokines with high sensitivity. Additionally, the bead-based format minimizes cross-reactivity between antibodies, ensuring high specificity. These assays are ideal for complex biological samples, such as serum, plasma, or cell culture supernatants, where multiple cytokines are present at varying concentrations.
- Customization with Cytokine Panels: The Luminex system offers pre-made cytokine panels tailored to specific research areas, such as inflammation, cancer, or immune response. These panels provide researchers with a ready-made solution for analyzing a set of cytokines relevant to their study, increasing efficiency and reducing the need for custom assay development.
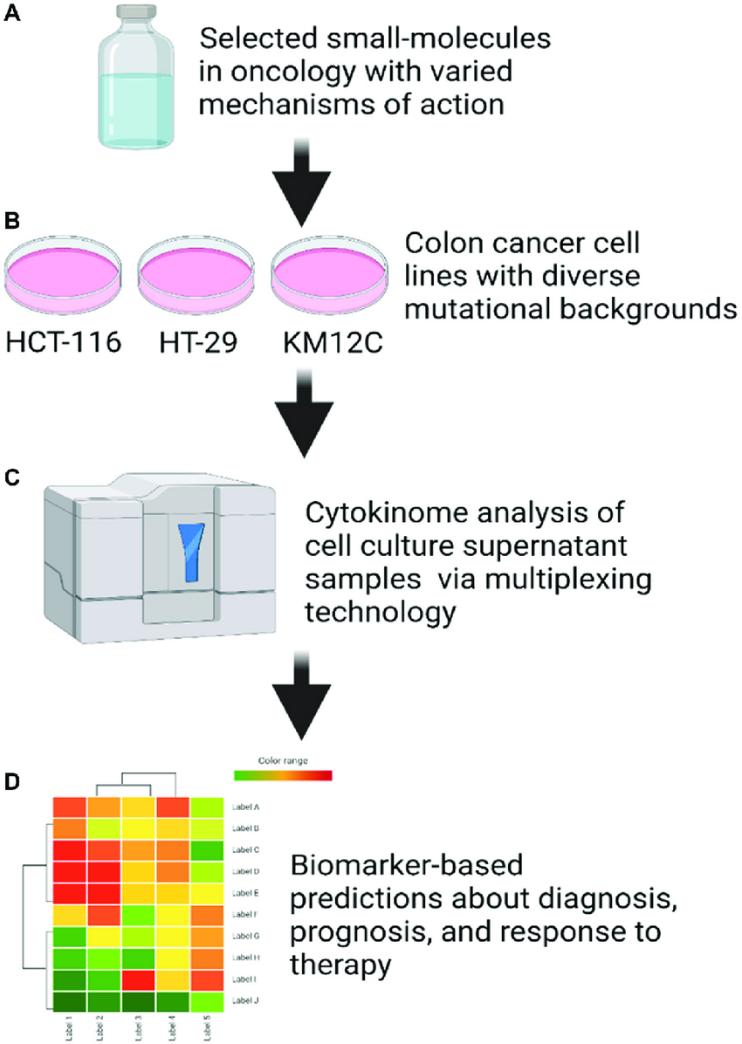 Cell culture supernatant cytokinome analysis workflow (Huntington, Kelsey E., et al., 2021).
Cell culture supernatant cytokinome analysis workflow (Huntington, Kelsey E., et al., 2021).
Flow Cytometry and Cytokine Bead Arrays
Flow cytometry is another powerful tool for cytokine analysis, particularly when combined with cytokine bead arrays. In this method, cytokines are captured on beads and analyzed using flow cytometry. The cytokines are detected through a fluorescently labeled detection antibody, and the fluorescence intensity is proportional to the amount of cytokine present.
- Bead-Based Assays: The advantage of cytokine bead arrays over traditional flow cytometry assays is that they allow for the simultaneous analysis of multiple cytokines. This provides a more comprehensive analysis of cytokine profiles in complex biological samples. Bead arrays can be adapted for different cytokines or immune markers depending on the specific research focus.
Data Analysis and Interpretation
Accurate data analysis is essential for interpreting cytokine results. In multiplex assays, data are typically collected from a flow cytometer or a Luminex analyzer, with cytokine concentrations quantified based on the fluorescent signals.
- Standard Curves: For quantification, a standard curve is generated using known concentrations of the cytokine of interest. The cytokine concentrations in experimental samples are then extrapolated from this curve.
- Normalization: Data normalization is necessary to account for variations between samples. This may include normalizing cytokine concentrations to total protein levels or cell counts to ensure that observed changes are not due to variations in sample volume or cell density.
- Statistical Analysis: Statistical methods are applied to assess the significance of differences in cytokine levels between experimental groups. Advanced tools, such as software from the Luminex platform or other bioinformatics tools, can be used to process and analyze complex data sets.
Best Practices for Cytokine Assays in Cell Culture
| Practice | Details |
|---|---|
| 1. Standardization of Sample Collection | - Consistent Timing: Cytokine secretion is time-dependent. Sampling at consistent time points ensures reliable data. - Cell Density: Standardize cell seeding density to avoid confounding results. High or low density may alter cytokine production. - Minimize Contamination: Use sterile techniques to prevent contamination that could affect assay outcomes. |
| 2. Optimization of Cytokine Detection Sensitivity | - Choice of Detection Method: Luminex xMAP technology allows sensitive multiplex detection. Ensure optimal bead performance for accurate readings. - Assay Sensitivity: Know the minimum detectable concentration (MDC) for each cytokine. Use sample concentration techniques if necessary. - Control Samples: Include positive and negative controls in every experiment to validate assay sensitivity and specificity. |
| 3. Minimizing Experimental Variability | - Uniform Stimulation Conditions: Apply consistent stimulator concentrations and incubation times across all samples to reduce variability. - Replicates: Use biological and technical replicates for accurate statistical analysis. - Avoiding Batch Effects: Standardize reagents and calibrate instruments to minimize batch-to-batch variation. |
| 4. Cytokine Panel Selection and Customization | - Pre-Made Panels: Commercial panels offer convenience and have been optimized for specific research areas, such as inflammation or immune response. - Custom Panels: For specialized studies, design custom panels to target a relevant cytokine profile, such as interleukins or growth factors. - Panel Design: Choose cytokines relevant to your experimental model, ensuring they cover the necessary signaling pathways and interactions. |
| 5. Data Normalization and Statistical Analysis | - Normalization: Normalize cytokine concentrations to cell numbers or total protein content to account for culture variations. - Statistical Analysis: Use statistical tests like ANOVA or non-parametric tests for evaluating group differences. Perform power analysis for optimal sample sizes. - Multiple Comparisons: Apply corrections for multiple comparisons (e.g., Bonferroni correction) to avoid Type I errors. |
| 6. Validation and Cross-Platform Comparison | - Validation: Cross-validate results using multiple methods (e.g., Luminex vs. ELISA) to confirm findings. - Comparison Across Platforms: Ensure consistency between platforms by comparing cytokine levels obtained from different assays, particularly for low-concentration cytokines. |
Applications of Cytokine Analysis in Cell Culture Research
Immune Response and Inflammation Studies
Cytokine analysis is indispensable for evaluating immune activation and inflammatory pathways in cultured cells. By quantifying cytokines such as TNF-α, IL-6, and IL-1β, researchers monitor inflammatory status and identify triggers of dysregulated immune responses.
- Mechanistic Insights: Profiling cytokines helps map signaling pathways (e.g., NF-κB, MAPK) involved in immune modulation.
- Multiplex Assays: Technologies like Luminex allow simultaneous measurement of multiple cytokines, enabling comprehensive tracking of immune activation in response to stimuli like pathogens or drugs.
Disease Modeling and Pathogenesis
Cytokine assays are pivotal for studying immune dysregulation in autoimmune disorders, chronic inflammation, and infectious diseases.
- Autoimmunity: Elevated pro-inflammatory cytokines (e.g., IL-17, IFN-γ) in cell cultures mimic autoimmune microenvironments, aiding in drug screening.
- Infection Models: Quantifying cytokines like IL-12 and IFN-γ reveals host-pathogen interactions and immune evasion strategies.
Cancer Research and Tumor Microenvironment
Cytokines shape tumor-immune crosstalk, influencing progression, metastasis, and therapy resistance.
- Immune Evasion: Tumor-derived cytokines (e.g., IL-10, TGF-β) suppress anti-cancer immunity; their detection helps evaluate immunotherapies.
- Microenvironment Profiling: Cytokine analysis in co-culture systems (tumor + immune cells) deciphers how cytokines like VEGF or IL-8 foster immunosuppressive niches.
Stem Cell Differentiation and Regenerative Medicine
Cytokines guide stem cell fate decisions and tissue repair mechanisms.
- Lineage Specification: Cytokines such as BMPs and Wnt ligands drive pluripotent stem cells toward neuronal, cardiac, or osteogenic lineages.
- Tissue Regeneration: Assays measuring HGF or FGF effects on cell proliferation aid in optimizing regenerative protocols.
Neuroinflammation and Neurodegeneration
Cytokine profiling in neural cell cultures elucidates mechanisms of diseases like Alzheimer's and Parkinson's.
- Glial Activation: Microglia and astrocytes secrete TNF-α and IL-1β during neuroinflammation; their levels correlate with neuronal damage.
- Neuronal Survival: Cytokine imbalances (e.g., elevated IFN-γ) are linked to synaptic dysfunction, guiding neuroprotective therapies.
Vaccine Development and Immune Profiling
Cytokine assays assess vaccine-induced immune responses and adjuvant efficacy.
- Th1/Th2 Balance: Dominance of IFN-γ (Th1) vs. IL-4 (Th2) cytokines informs vaccine design for targeted immunity.
- Adjuvant Screening: Cytokine signatures (e.g., IL-2, IL-6) validate adjuvant potency in enhancing antigen-specific responses.
Infectious Disease and Antiviral Responses
Cytokine storms and immune evasion strategies are studied using pathogen-exposed cell cultures.
- Viral Infections: Upregulation of IFN-α/β and IL-6 highlights antiviral defense mechanisms.
- Bacterial Models: Pro-inflammatory cytokines (e.g., IL-1β) signal hyperactivation in sepsis or chronic infections.
Reference:
- Huntington, Kelsey E., et al. "A high-throughput customized cytokinome screen of colon cancer cell responses to small-molecule oncology drugs." Oncotarget 12.20 (2021): 1980. https://doi.org/10.18632/oncotarget.28079

