Definition of Regulatory T Cells (Tregs)
Regulatory T cells (Tregs) are a specialized subset of T cells that play a crucial role in maintaining immune system homeostasis and preventing autoimmune responses. They are primarily characterized by the expression of the transcription factor Forkhead box P3 (FoxP3), which is essential for their development and regulatory functions. Tregs are phenotypically identified by their expression of CD4, CD25 (the alpha chain of the IL-2 receptor), and low levels of CD127 (the IL-7 receptor alpha chain).
Types of Tregs
Tregs can be broadly classified into two main categories based on their origin:
Natural Tregs (nTregs)
Natural Tregs, also known as thymus-derived Tregs, develop directly in the thymus during the process of T cell maturation. These cells are positively selected in the thymus for their ability to recognize self-antigens with high affinity, a process essential for establishing central tolerance. nTregs play a crucial role in maintaining self-tolerance and preventing autoimmune reactions by suppressing autoreactive T cells that escape thymic deletion.
Induced Tregs (iTregs)
Induced Tregs, also known as peripheral Tregs, originate from conventional CD4+ T cells in the periphery under specific conditions. The differentiation of iTregs is induced by various environmental signals, including the presence of certain cytokines like TGF-β and IL-2, as well as interactions with antigen-presenting cells. iTregs are vital for maintaining immune tolerance in peripheral tissues and for modulating immune responses to non-self antigens, such as those from commensal microbiota or dietary components.
What is The Difference Between T cells and Tregs?
| Feature | T Cells | Tregs (Regulatory T Cells) |
|---|---|---|
| Function | Recognize and respond to pathogens, infected cells, or cancer cells. | Regulate and suppress immune responses to maintain balance and prevent autoimmunity. |
| Types/Subsets | Helper T cells (Th), Cytotoxic T cells (Tc), Memory T cells, etc. | CD4+CD25+ Tregs, FoxP3+ Tregs |
| Primary Role | Activate immune responses, kill infected or abnormal cells, provide long-term immunity. | Inhibit excessive or inappropriate immune responses, prevent autoimmunity. |
| Activation | Activated by antigen presentation and interaction with antigen-presenting cells (APCs). | Activated by self-antigens or specific immune signals to suppress other T cell activities. |
| Surface Markers | Varies by subset; common markers include CD3, CD4, CD8. | Common markers include CD4, CD25, and FoxP3. |
| Impact on Immune System | Enhances immune responses against infections and tumors. | Dampens immune responses to maintain tolerance and prevent autoimmunity. |
Mechanisms of Treg Action
Tregs are essential for maintaining immune tolerance and preventing autoimmune diseases through a combination of sophisticated mechanisms. These mechanisms can be categorized broadly into cell-cell contact-dependent suppression, cytokine-mediated suppression, and metabolic disruption, each playing a crucial role in modulating immune responses.
Cell-cell contact-dependent suppression involves direct interactions between Tregs and other immune cells, primarily through surface molecules like CTLA-4, LAG-3, and TIGIT. CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4) is a key inhibitory molecule expressed on Tregs that competes with CD28 for binding to CD80 and CD86 on antigen-presenting cells (APCs). By binding to these ligands, CTLA-4 transmits inhibitory signals to APCs, reducing their ability to activate effector T cells, thus dampening the immune response. Similarly, LAG-3 (Lymphocyte-activation gene 3) binds to MHC class II molecules on APCs, inhibiting their activation and cytokine production, which in turn reduces the activation and proliferation of effector T cells. TIGIT (T cell immunoreceptor with Ig and ITIM domains) interacts with CD155 on APCs, delivering suppressive signals that inhibit the activation and function of both APCs and effector T cells.
Cytokine-mediated suppression is another crucial mechanism employed by Tregs. They secrete various anti-inflammatory cytokines, including IL-10, TGF-β, and IL-35. IL-10 inhibits the production of pro-inflammatory cytokines by effector T cells and macrophages and reduces the antigen-presenting capacity of dendritic cells, leading to decreased T cell activation and proliferation. TGF-β induces the expression of FoxP3, promoting the development of Tregs, and suppresses the proliferation and differentiation of effector T cells, contributing to the maintenance of immune tolerance. IL-35, a heterodimeric cytokine composed of IL-12p35 and Ebi3 subunits, promotes the expansion of Tregs and enhances their suppressive activity. It inhibits the proliferation of effector T cells and the production of pro-inflammatory cytokines, thereby reducing inflammation and autoimmunity.
Metabolic disruption is another vital mechanism through which Tregs influence the immune response. Tregs express high levels of CD25, the alpha chain of the IL-2 receptor, allowing them to consume IL-2 rapidly. By sequestering IL-2, Tregs deprive effector T cells of this critical growth factor, limiting their proliferation and function. Additionally, Tregs express ectonucleotidases such as CD39 and CD73, which convert ATP and ADP to adenosine, a potent immunosuppressive molecule. Adenosine acts on adenosine receptors on effector T cells and other immune cells, inhibiting their activation and promoting a suppressive microenvironment.
Furthermore, Tregs can modulate the function of dendritic cells (DCs) to enforce immune tolerance. Through interactions with dendritic cells, Tregs maintain them in an immature state. Immature DCs have a reduced capacity to activate effector T cells and are more prone to induce tolerance. Tregs promote this state through the secretion of cytokines like IL-10 and TGF-β and direct cell-cell interactions.
Select Service
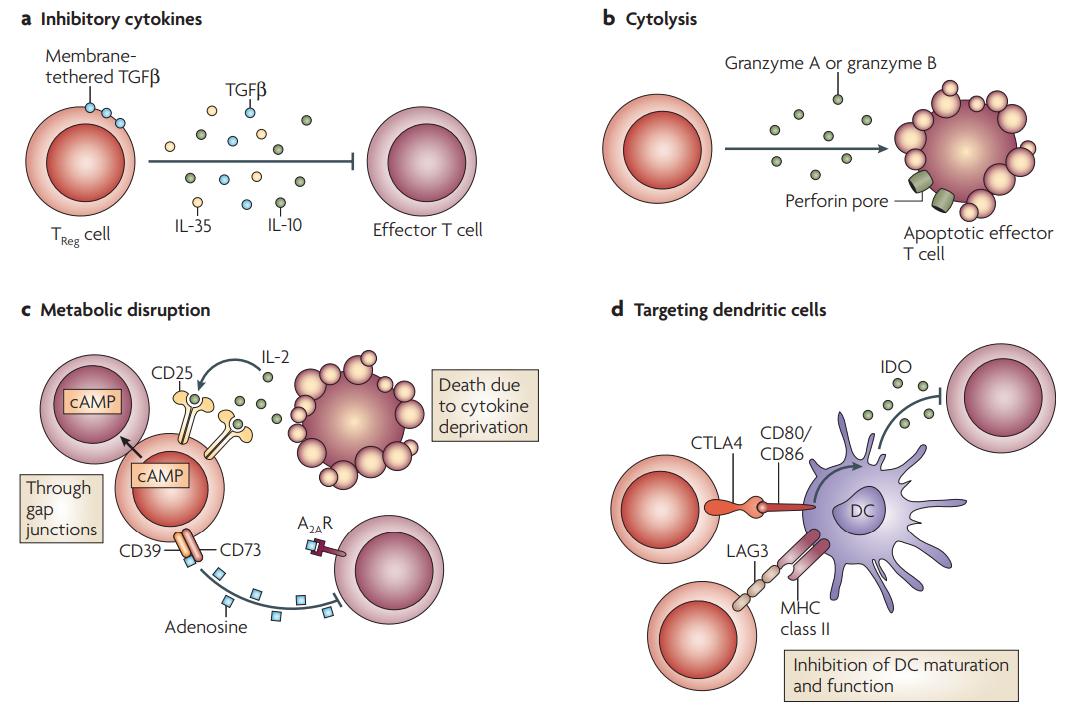 Basic mechanisms used by TReg cells (Vignali et al., 2008).
Basic mechanisms used by TReg cells (Vignali et al., 2008).
Function of Regulatory T Cells (Tregs)
Maintaining Immune Tolerance: Tregs help prevent the immune system from attacking the body's own tissues, thereby maintaining self-tolerance. This is vital for preventing autoimmune diseases, where the immune system mistakenly targets and damages healthy tissues.
Suppressing Autoimmune Responses: Tregs inhibit the activation and proliferation of autoreactive T cells that escape central tolerance mechanisms in the thymus. By suppressing these potentially harmful cells, Tregs protect the body from autoimmune reactions.
Modulating Immune Responses: Tregs regulate the magnitude and duration of immune responses to prevent excessive inflammation and tissue damage. This modulation is crucial during infections and inflammatory conditions to ensure that immune responses are effective yet controlled.
Facilitating Immune Resolution: After an infection or inflammatory response, Tregs help to resolve inflammation and promote the return to homeostasis. They do this by suppressing the activity of effector T cells and other immune cells involved in the inflammatory response.
Promoting Tissue Repair and Healing: By controlling excessive immune responses and inflammation, Tregs create an environment conducive to tissue repair and healing. They help to minimize collateral damage to tissues during immune responses.
Regulating Responses to Non-Self Antigens: Tregs are involved in controlling immune responses to non-self antigens, such as those from commensal microbiota and dietary components. This helps maintain a balanced relationship with beneficial microorganisms and prevents inappropriate immune reactions to harmless antigens.
Supporting Transplantation Tolerance: Tregs play a significant role in promoting tolerance to transplanted organs and tissues. By suppressing immune responses against the transplanted material, Tregs help prevent transplant rejection and improve graft survival.
Influencing Tumor Immunity: In the context of cancer, Tregs can suppress anti-tumor immune responses, which can allow tumors to evade immune surveillance. This dual role makes Tregs a target of interest in cancer immunotherapy, where their activity might be modulated to enhance anti-tumor immunity.
Key Cytokines Produced by Tregs
IL-10 is produced by Tregs in response to various stimuli, including interactions with antigen-presenting cells (APCs) and exposure to certain antigens.
Mechanisms and Functions:
- Anti-inflammatory Effects: IL-10 is a potent anti-inflammatory cytokine that inhibits the production of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and TNF-α by macrophages and dendritic cells. This helps to reduce inflammation and prevent tissue damage during immune responses.
- Inhibition of Antigen Presentation: IL-10 reduces the expression of major histocompatibility complex (MHC) class II molecules and co-stimulatory molecules on APCs. This diminishes their ability to present antigens and activate effector T cells, thus dampening the immune response.
- Suppression of T Cell Activity: IL-10 directly inhibits the proliferation and cytokine production of effector T cells, including Th1 and Th17 cells, which are often involved in inflammatory and autoimmune responses.
Transforming Growth Factor-beta (TGF-β)
TGF-β is secreted by Tregs and is also present in its latent form bound to the extracellular matrix. Activation of TGF-β requires proteolytic cleavage or interaction with integrins.
Mechanisms and Functions:
- Promotion of Treg Differentiation: TGF-β is crucial for the differentiation of induced Tregs (iTregs) from conventional CD4+ T cells in the periphery. It induces the expression of FoxP3, the transcription factor essential for Treg development and function.
- Suppression of Effector T Cells: TGF-β inhibits the proliferation and differentiation of effector T cells, including Th1 and Th2 cells. It also inhibits the differentiation of Th17 cells by antagonizing the effects of IL-6 and IL-23.
- Regulation of Immune Cell Function: TGF-β affects various immune cells, including macrophages, dendritic cells, and B cells. It promotes a regulatory phenotype in macrophages and dendritic cells, enhancing their ability to support Treg function and further suppress immune responses.
IL-35 is a heterodimeric cytokine composed of the subunits IL-12p35 and Epstein-Barr virus-induced gene 3 (Ebi3). It is specifically produced by Tregs and not by other immune cells under normal conditions.
Mechanisms and Functions:
- Enhancement of Treg Function: IL-35 promotes the proliferation and suppressive function of Tregs, creating a positive feedback loop that enhances Treg-mediated immune regulation.
- Inhibition of Effector T Cell Proliferation: IL-35 directly suppresses the proliferation of effector T cells, including both CD4+ and CD8+ T cells, reducing their ability to mount an effective immune response.
- Induction of Regulatory T Cell Population: IL-35 can induce the formation of a unique population of regulatory T cells known as IL-35-induced regulatory T cells (iTr35 cells). These cells possess potent suppressive capabilities and contribute to the overall regulatory milieu.
| Species | Description | Analytes | |
|---|---|---|---|
| Human | Human Treg 7-Plex Panel | IL-9、IL-10、IL-17A、IL-21、IL-22、IL-23、IL-27 | +Inquiry |
| Human Treg 12-Plex Panel | IL-2、1L-10、IL-12(p40)、IL-12(p70)、IL-19、IL-20、IL-22、1L-26、1L-27(28)、IL-28A/IFN-λ2、IL-29/IFN-λ1, IL-35 | +Inquiry | |
| Human Treg 14-Plex Panel | INFα、IL-1α、ILIRA、IL-7、IL9、IL-10、IL-15、IL-17A、IL-21、IL-22、IL-23、IL-27、IL-31、TNFβ | +Inquiry | |
| Human Treg 16-Plex Panel | Eotaxin、GROα/CXCL1、IL-8/CXCL8、IL-9、IL-10、IL-17A、IL-21、IL-22、IL-23、IL-27、IP-10/CXCL10、MCP-1/CCL2、MCP-1/CCL3、MIP-1β/CCL4、Rantes、SDF1α/CXCL12 | +Inquiry | |
| Mouse | Mouse Treg Cytokine 6-Plex Panel | IL-9, IL-10, IL-17A, IL-22, IL-23, IL-27 | +Inquiry |
| Mouse Treg Cytokine 15-Plex Panel | IL-10, IL-17A, IL-22, IL-23, IL-27, IL-9, GROα, IP-10, MCP-1, MCP-3, MIP-1α, MIP-1β, MIP-2, RANTES, Eotaxin | +Inquiry | |
| Mouse Treg Cytokine 17-Plex Panel | GM-CSF, IFNγ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-18, IL-22, IL-23, IL-27, TNFα | +Inquiry |
Key Proteins Associated with Tregs
FoxP3 (Forkhead Box P3)
FoxP3 is the master transcription factor essential for the development and function of Tregs. It is crucial for their identity and suppressive capabilities.
Mechanisms:
- Gene Regulation: FoxP3 regulates the expression of genes necessary for Treg function, including those involved in suppressive signaling and immune tolerance. It promotes the expression of various inhibitory molecules and cytokines.
- Development: FoxP3 is critical for the differentiation of Tregs in the thymus (natural Tregs) and in peripheral tissues (induced Tregs). Mutations or deficiencies in FoxP3 lead to severe autoimmune disorders, such as IPEX syndrome, underscoring its essential role.
CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4)
CTLA-4 is a co-inhibitory receptor expressed on the surface of Tregs, playing a key role in suppressing immune responses.
Mechanisms:
- Competition with CD28: CTLA-4 competes with the costimulatory molecule CD28 for binding to CD80 and CD86 on antigen-presenting cells (APCs). By outcompeting CD28, CTLA-4 inhibits the activation of effector T cells and reduces their proliferation.
- Signal Transduction: Engagement of CTLA-4 with its ligands delivers inhibitory signals to both Tregs and APCs, leading to a decrease in T cell activation and cytokine production.
LAG-3 (Lymphocyte-activation gene 3)
LAG-3 is another inhibitory receptor expressed on Tregs that modulates immune responses.
- Binding to MHC Class II: LAG-3 binds to MHC class II molecules on APCs. This interaction inhibits the activation of APCs and reduces their ability to stimulate effector T cells.
- Inhibition of T Cell Activation: LAG-3 signaling suppresses T cell activation and cytokine production, contributing to the overall suppressive function of Tregs.
TIGIT (T cell immunoreceptor with Ig and ITIM domains)
TIGIT is an inhibitory receptor on Tregs that plays a role in immune regulation.
Mechanisms:
- Interaction with CD155: TIGIT binds to CD155 (also known as PVR) on APCs and other cells. This interaction delivers inhibitory signals that reduce the activation and function of both APCs and effector T cells.
- Regulation of T Cell Responses: By interacting with CD155, TIGIT helps to maintain immune tolerance and prevent excessive immune responses.
CD39 and CD73
CD39 and CD73 are ectonucleotidases expressed on the surface of Tregs that contribute to their suppressive function through the production of adenosine.
Mechanisms:
- ATP Degradation: CD39 converts ATP to ADP and then to AMP. Subsequently, CD73 converts AMP to adenosine.
- Adenosine Production: Adenosine is a potent immunosuppressive molecule that acts on adenosine receptors on effector T cells and other immune cells. It inhibits T cell activation, proliferation, and cytokine production, thereby promoting a suppressive environment.
GITR (Glucocorticoid-Induced Tumor Necrosis Factor Receptor)
GITR is expressed on Tregs and modulates their activity. It can enhance Treg proliferation and function when engaged. GITR signaling can alter the suppressive capacity of Tregs and impact their interactions with other immune cells.
PD-1 (Programmed Death-1)
PD-1 is an inhibitory receptor expressed on Tregs that contributes to immune regulation. PD-1 interacts with its ligands PD-L1 and PD-L2 on APCs, delivering inhibitory signals that further suppress T cell activity and maintain immune tolerance.
Luminex Panel Method for Analyzing Tregs
The Luminex panel is a powerful multiplex technology used to measure multiple cytokines, proteins, or other biomarkers in a single sample. It is particularly useful for analyzing Tregs in research and clinical settings, as it allows for the simultaneous detection and quantification of various cytokines and proteins associated with Treg function.
Key Features:
- Multiplexing Capability: Measures multiple cytokines or proteins in a single assay.
- Quantitative Data: Provides precise concentration measurements of each analyte.
- Flexible Panels: Customizable to include a range of analytes relevant to Treg research.
Applications
Cytokine Profiling: Measure levels of cytokines such as IL-10, TGF-β, and IL-35 produced by Tregs to assess their regulatory functions and immunosuppressive properties.
Protein Expression: Analyze the expression of proteins associated with Treg activity, such as CTLA-4, CD39, and CD73, which can provide insights into their functional status and role in disease.
Immune Monitoring: Evaluate changes in cytokine profiles in response to therapies or disease progression, which can help in understanding the impact of Tregs on immune regulation.
Reference:
- Vignali, Dario AA, Lauren W. Collison, and Creg J. Workman. "How regulatory T cells work." Nature reviews immunology 8.7 (2008): 523-532.