Overview of Cytokines
Cytokines are a diverse group of small signaling proteins that play essential roles in modulating immune responses, inflammation, and cell communication. These molecules act through specific receptors, triggering intracellular signaling cascades that influence cell survival, proliferation, and differentiation.
Classification of Cytokines
Cytokines can be classified based on their function and target effects:
- Pro-inflammatory cytokines: TNF-α, IL-6, IL-1β, IFN-γ
- Anti-inflammatory cytokines: IL-10, TGF-β
- Growth and hematopoietic factors: GM-CSF, EPO
- Chemokines: CXCL8 (IL-8), CCL2
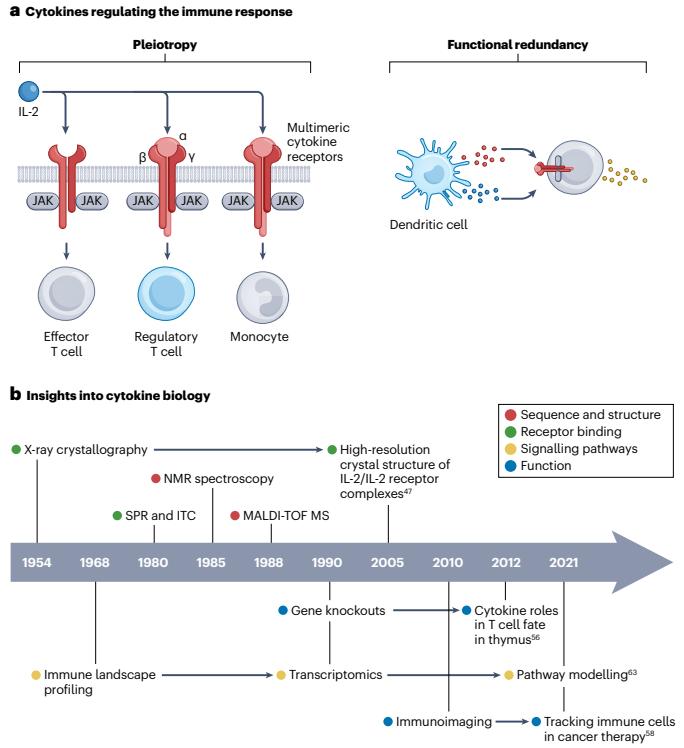 Cytokine biology and mechanisms (Deckers, Jeroen, et al. 2023).
Cytokine biology and mechanisms (Deckers, Jeroen, et al. 2023).
The Role of Cytokines in Drug Development
Cytokines are integral to drug discovery and development, with their applications spanning:
- Target identification and validation for autoimmune and inflammatory diseases
- Development of cytokine-based therapeutics (e.g., IL-2 in cancer immunotherapy)
- Biomarker discovery for personalized medicine
- Monitoring treatment efficacy and immune responses in clinical trials
Cytokine Analysis in Biopharmaceutical Research
Cytokine profiling is critical in drug development for:
- Identifying immune-related adverse effects
- Understanding the mechanism of action of immunomodulatory drugs
- Predicting therapeutic responses in patients
Key Areas of Cytokine Research in Drug Development
Autoimmune and Inflammatory Disease Therapeutics
Autoimmune and inflammatory diseases are characterized by dysregulated immune responses, often driven by imbalances in cytokine signaling. These conditions, including rheumatoid arthritis (RA), inflammatory bowel disease (IBD), systemic lupus erythematosus (SLE), and psoriasis, involve excessive activation of pro-inflammatory pathways and inadequate regulatory mechanisms. Targeting cytokines in these diseases has revolutionized treatment strategies, leading to the development of biologics and small-molecule inhibitors.
Cytokines in Inflammatory Responses
Cytokines are crucial mediators of inflammation, orchestrating the recruitment and activation of immune cells. The balance between pro-inflammatory and anti-inflammatory cytokines determines disease progression and treatment efficacy.
Pro-inflammatory cytokines:
- TNF-α: A master regulator of inflammation, TNF-α drives synovial inflammation and joint destruction in RA. It is also implicated in Crohn's disease by promoting intestinal barrier dysfunction.
- IL-6: Plays a pivotal role in immune cell differentiation and acute-phase responses. Elevated IL-6 levels correlate with disease severity in RA and contribute to systemic inflammation.
- IL-1β: A key mediator of inflammasome activation, IL-1β is involved in fever, pain, and chronic inflammation.
Anti-inflammatory cytokines:
- IL-10: A potent immunosuppressive cytokine that inhibits pro-inflammatory responses and promotes tissue repair. Its deficiency is linked to severe colitis and autoimmune disorders.
- TGF-β: Regulates immune tolerance by promoting regulatory T cell (Treg) differentiation and inhibiting excessive immune activation.
Cytokine-Targeted Therapies
Several biologics and small-molecule drugs have been developed to modulate cytokine activity in autoimmune and inflammatory diseases:
- TNF inhibitors:
Infliximab and etanercept are monoclonal antibodies or receptor fusion proteins that neutralize TNF-α, significantly improving symptoms in RA, IBD, and psoriasis.
- IL-6 receptor antagonists:
Tocilizumab, an IL-6 receptor-blocking antibody, has demonstrated efficacy in treating RA and cytokine storm syndromes.
- JAK/STAT inhibitors:
Small molecules like tofacitinib and baricitinib inhibit cytokine-driven JAK/STAT signaling, reducing inflammation in RA and ulcerative colitis.
By understanding cytokine networks, researchers continue to refine therapeutic strategies to achieve greater specificity and reduced immunosuppression-related side effects.
Cytokines in Cancer Immunotherapy
Cytokines play dual roles in cancer biology, acting as either tumor-promoting or tumor-suppressing factors. The tumor microenvironment (TME) is a complex ecosystem where cytokines regulate immune cell infiltration, tumor cell survival, and resistance to therapy.
The Dual Role of Cytokines in Tumors
Immunosuppressive cytokines:
- TGF-β: Promotes tumor progression by suppressing cytotoxic T lymphocyte (CTL) activity and enhancing tumor-associated fibroblast expansion.
- IL-10: Inhibits antigen-presenting cell function and supports regulatory T cell (Treg) activity, facilitating immune evasion.
Pro-inflammatory cytokines:
- IFN-γ: Enhances tumor antigen presentation and activates NK cells and CTLs to attack tumor cells.
- IL-2: Stimulates T cell proliferation and has been used as a cancer immunotherapy agent.
Cytokine-Based Cancer Therapies
Several cytokine-targeted strategies are currently in clinical use or development:
Cytokine monotherapies:
- High-dose IL-2 therapy has been FDA-approved for metastatic melanoma and renal cell carcinoma.
- IFN-α has been utilized to enhance anti-tumor immune responses in hematologic malignancies.
Cytokines in CAR-T Cell Therapy:
- IL-15: Enhances CAR-T cell expansion and persistence, improving long-term tumor clearance.
- Engineering CAR-T cells to secrete cytokines (e.g., IL-12) enhances local immune activation while reducing systemic toxicity.
Cytokine modulation in checkpoint blockade therapy:
- Combining anti-PD-1 therapy with cytokine modulation enhances T cell infiltration and response rates in solid tumors.
Cytokine Storms and Infectious Diseases
Cytokine storms refer to the uncontrolled and excessive release of pro-inflammatory cytokines, often seen in severe infections such as COVID-19, SARS, and influenza. These overwhelming immune responses can lead to systemic inflammation, multi-organ failure, and death.
Cytokine Storm Pathophysiology
Cytokine storms are driven by:
- Excessive TNF-α, IL-6, and IL-1β production, which amplifies immune cell recruitment and inflammation.
- Dysregulated interferon responses, where delayed or excessive IFN signaling contributes to viral persistence and tissue damage.
Therapeutic Strategies for Cytokine Storms
Corticosteroids: Dexamethasone reduces inflammation and mortality in severe viral pneumonia.
JAK inhibitors: Baricitinib dampens cytokine overproduction by targeting JAK1/2 signaling.
Cytokines in Vaccine Development
Cytokines are also crucial in shaping vaccine-induced immunity:
- IL-12 promotes Th1 responses, enhancing cellular immunity against intracellular pathogens.
- GM-CSF acts as an adjuvant, boosting antigen-presenting cell activation and vaccine efficacy.
Targeted cytokine modulation is essential for designing safer and more effective therapies against infectious diseases.
Cytokine Engineering for Drug Development
Cytokine engineering enhances therapeutic efficacy while minimizing toxicity. Strategies include modified cytokines, conjugated therapies, and targeted delivery systems.
Modified Cytokines: Enhancing Efficacy and Reducing Toxicity
- Pegylated Cytokines: PEGylation (attachment of polyethylene glycol) enhances cytokine stability and prolongs circulation time. Pegylated IL-2 (Bempegaldesleukin) is designed to selectively activate effector T cells while reducing regulatory T cell activation, improving its efficacy in cancer immunotherapy.
- Cytokine Mutants: Site-directed mutagenesis has been used to create cytokine variants with altered receptor binding affinity, leading to improved specificity and reduced systemic side effects. Engineered IL-2 variants, such as THOR-707, selectively stimulate cytotoxic T cells without activating immunosuppressive Tregs.
Cytokine-Conjugated Therapies: Improving Targeting and Delivery
To enhance specificity and minimize toxicity, cytokines can be conjugated with other therapeutic agents, such as monoclonal antibodies, nanoparticles, or fusion proteins:
- Cytokine-Antibody Fusion Proteins (Immunocytokines): These molecules combine cytokines with tumor-targeting antibodies to enhance immune activation in the tumor microenvironment while reducing systemic inflammation. For example, L19-IL2 fuses IL-2 with a tumor-targeting antibody to improve anti-tumor responses.
- Nanoparticle-Based Cytokine Delivery: Encapsulating cytokines within nanoparticles allows for controlled release, increased stability, and improved targeting. Liposomal IL-12 formulations have shown promise in boosting anti-cancer immunity while reducing systemic toxicity.
Targeted Cytokine Delivery Systems: Enhancing Precision Medicine
Advancements in targeted delivery aim to localize cytokine action at disease sites, reducing off-target effects:
- Prodrug Cytokines: These inactive cytokine precursors are designed to be activated only in specific tissues or under certain enzymatic conditions. For example, tumor microenvironment-activated IL-12 prodrugs enhance immune responses in tumors without triggering systemic toxicity.
- Gene Therapy Approaches: Cytokine-encoding gene therapy vectors, such as oncolytic viruses engineered to express IL-12 or GM-CSF, offer localized and sustained cytokine production in tumors.
Cytokine Analysis in Drug Development
Cytokines, as central mediators of immune and inflammatory regulation, have made their dynamic monitoring and analysis technologies indispensable in modern drug development. From target validation to clinical translation, cytokine analysis permeates the entire drug development cycle.
Cytokine Analysis Technology
High-Throughput Multi-Factor Detection Platforms
Traditional methods such as ELISA (single-factor detection) and Western Blot (protein expression verification) are straightforward but struggle to resolve complex cytokine networks. Recently, Luminex xMAP technology has enabled the parallel detection of over 50 cytokines in a single sample using fluorescently encoded beads, with sensitivity reaching pg/mL levels and requiring as little as 25 μL of sample. For example, AstraZeneca used Luminex multiplex detection to analyze 48 factors simultaneously during the development of anti-IL-33 antibodies, quickly identifying IL-5 and IL-13 as companion diagnostic biomarkers.
We can also provide other customized panels, contact us to learn more!
Single-Cell Resolution Techniques
Flow cytometry (FACS) combined with intracellular staining can resolve the cytokine secretion profiles of T-cell and macrophage subpopulations at the single-cell level. For instance, in CAR-T therapy development, detecting IL-15 secretion levels helped screen for high-persistence T-cell clones, increasing the complete remission rate by 30%.
Services you may be interested in:
Applications Across the Drug Development Cycle
Early Drug Screening and Mechanism Validation
- Target Discovery: Identifying key regulatory nodes through cytokine network analysis. For example, IL-1β was confirmed as a core target for atherosclerosis, and its inhibitor Canakinumab reduced cardiovascular event risk by 15%.
- In Vitro Model Optimization: Monitoring IL-22 levels in 3D organoid models to assess tissue-specific effects of intestinal regeneration drugs.
Preclinical Safety Assessment
- Cytokine Storm Warning: Dynamically monitoring IL-6 and IFN-γ levels in CAR-T animal models can predict immune toxicity. An IL-6 peak >100 pg/mL indicates a high risk of severe CRS, necessitating adjustments to dosing regimens.
- Off-Target Effect Analysis: Engineered IL-2 mutants (like THOR-707) reduce systemic toxicity by decreasing Treg cell activation, with safety validation dependent on parallel detection of IL-10 and TGF-β.
Clinical Trial Optimization and Precision Stratification
- Efficacy Prediction: Baseline serumIL-8 levels >20 pg/mL in melanoma patients resulted in a response rate of only 7% to PD-1 inhibitors, while lower levels achieved a 35% response rate.
- Dose Adjustment: Dynamically monitoring IL-6 levels during the use of tocilizumab (anti-IL-6R) in severe COVID-19 patients optimized dosing intervals, reducing the 28-day mortality rate from 33% to 29%.
Industry Challenges and Solutions
Breaking Technical Bottlenecks
- Detection of Low-Abundance Factors: Using ultra-sensitive microfluidic chips (detection limit of 0.1 pg/mL) for precise quantification of low-expression factors like IL-17A, aiding in psoriasis subtype diagnosis.
- Real-Time Dynamic Monitoring: Implantable biosensors can continuously monitor IL-12 and TGF-β fluctuations in the tumor microenvironment for up to 72 hours, guiding the timing of immunotherapy administration.
Data Standardization and Quality Control
Establishing cross-platform cytokine detection standardization processes (such as MIAME standards) and ensuring data comparability through Luminex technology's multiplex validation capabilities.
Reference:
- Deckers, Jeroen, et al. "Engineering cytokine therapeutics." Nature Reviews Bioengineering 1.4 (2023): 286-303.

