CD4+ T helper cells (Th cells) are an important component of the adaptive immune system, regulating immune responses by secreting specific cytokines. Based on the cytokines they secrete, CD4+ T cells can be divided into two main subtypes: Th1 and Th2. Th1 cells primarily participate in cell-mediated immunity, fighting against viruses and bacteria that are intracellular pathogens, while Th2 cells promote humoral immunity, defending against parasitic infections and playing a crucial role in allergic reactions.
What Are Th1 and Th2 Cytokines?
Th1 and Th2 cells produce specific cytokines that dictate the nature of immune responses:
Th1 Cytokines
Th1 cells are primarily involved in cellular immunity, helping to eliminate intracellular pathogens like viruses and bacteria. The major cytokines associated with Th1 responses include:
- Interferon-gamma (IFN-γ): Enhances macrophage activation and promotes antigen presentation.
- Tumor Necrosis Factor-alpha (TNF-α): Contributes to inflammation and immune cell recruitment.
- Interleukin-2 (IL-2): Supports T cell proliferation and activation.
Th2 Cytokines
Th2 cells are more associated with humoral immunity, helping to combat extracellular pathogens and parasites while playing a role in allergic reactions. Key Th2 cytokines include:
- Interleukin-4 (IL-4): Induces B cell differentiation and IgE production.
- Interleukin-5 (IL-5): Stimulates eosinophil growth and activation.
- Interleukin-10 (IL-10): Suppresses excessive immune responses to prevent tissue damage.
- Interleukin-13 (IL-13): Promotes mucus production and airway hyperreactivity.
👉 The Luminex Human Th1/Th2 Cytokine Panel provides a powerful tool for simultaneously measuring these key cytokines, allowing researchers to investigate immune response dynamics with high sensitivity and accuracy.
Th1 and Th2 Responses: What Are They?
Th1 Response: Guardians Against Intracellular Pathogens
Th1 differentiation is driven by IL-12 and IFN-γ signaling, activating the master transcription factor T-bet. This response specializes in eradicating intracellular invaders through three key mechanisms:
- Macrophage Activation: IFN-γ primes macrophages to enhance phagosomal acidification and generate reactive nitrogen intermediates, effectively killing Mycobacterium tuberculosis and Leishmania parasites.
- Cytotoxic T Cell Recruitment: Th1-derived IL-2 expands CD8+ T cell clones capable of lysing virus-infected cells (e.g., influenza, HIV).
- IgG Isotype Switching: Through CD40L co-stimulation, Th1 cells promote B cell production of opsonizing IgG2a/IgG3 antibodies critical for bacterial clearance.
- Pro-inflammatory Effects: Th1 cells drive chronic inflammation by recruiting neutrophils and monocytes to infection sites. In autoimmune diseases, excessive Th1 activity contributes to tissue damage, as seen in rheumatoid arthritis and multiple sclerosis.
A hallmark of Th1 dominance is granuloma formation—a macrophage/T cell conglomerate that walls off persistent pathogens like Histoplasma capsulatum.
Th2 Response: Defenders Against Extracellular Threats
GATA3-dependent Th2 polarization, triggered by IL-4 and thymic stromal lymphopoietin (TSLP), mounts defenses against helminths and allergens via:
- Eosinophil/Basophil Mobilization: IL-5 and IL-9 drive bone marrow eosinopoiesis and mast cell degranulation, respectively, enabling expulsion of Schistosoma mansoni larvae.
- Mucus Hyperproduction: IL-13 induces goblet cell hyperplasia and MUC5AC secretion, trapping Ascaris worms in intestinal mucin layers.
- IgE Class Switching: IL-4 directs B cells to produce IgE antibodies that coat Anisakis nematodes for eosinophil-mediated ADCC (antibody-dependent cellular cytotoxicity).
The Th2 cytokine triad (IL-4/IL-5/IL-13) also mediates tissue repair through alternative macrophage activation (M2 phenotype), though dysregulation leads to fibrotic pathologies.
Regulation of Th1/Th2 Balance
Key Factors Regulating Th1/Th2 Differentiation
Cytokine Environment
The cytokine milieu during T cell activation is the most critical factor determining whether a naive CD4+ T cell differentiates into a Th1 or Th2 cell.
- Th1 differentiation is promoted by IL-12 and IFN-γ, which activate STAT4 and T-bet transcription factors.
- Th2 differentiation is driven by IL-4, activating STAT6 and GATA3, leading to increased IL-5, IL-10, and IL-13 production.
Antigen Type and Presentation
- Intracellular pathogens (e.g., viruses, certain bacteria) trigger Th1 responses via dendritic cells and macrophages producing IL-12.
- Extracellular pathogens (e.g., helminths, allergens) favor Th2 differentiation, often through basophils, mast cells, and eosinophils releasing IL-4.
Genetic and Epigenetic Factors
- Genetic predisposition influences an individual's Th1/Th2 bias, with specific HLA alleles and cytokine gene polymorphisms affecting immune responses.
- Epigenetic modifications, such as DNA methylation and histone acetylation, regulate cytokine gene expression and Th cell differentiation.
Microbiota and Environmental Factors
- The gut microbiome plays a crucial role in shaping immune responses. Commensal bacteria can promote Th1 or Th2 immunity depending on the microbial composition.
- Environmental factors such as diet, pollutants, stress, and infections influence immune system development and Th1/Th2 balance.
- Hygiene hypothesis: Reduced early-life exposure to microbes may shift the immune system toward a Th2-dominant response, increasing allergy and asthma risks.
Th1/Th2 Imbalance and Disease
An improper Th1/Th2 balance can lead to immune dysfunction and disease:
- Th1-dominant conditions: Autoimmune diseases (e.g., type 1 diabetes, rheumatoid arthritis, multiple sclerosis) result from excessive cell-mediated immunity and chronic inflammation.
- Th2-dominant conditions: Allergic diseases (e.g., asthma, eczema, allergic rhinitis) and chronic parasitic infections arise due to excessive humoral immunity and eosinophilic inflammation.
Accurately measuring Th1 and Th2 cytokines is critical for understanding immune dysregulation. The Luminex Human Th1/Th2 Cytokine Panel provides high-throughput, multiplex analysis of key cytokines, enabling researchers to study immune balance in disease states and therapeutic interventions.
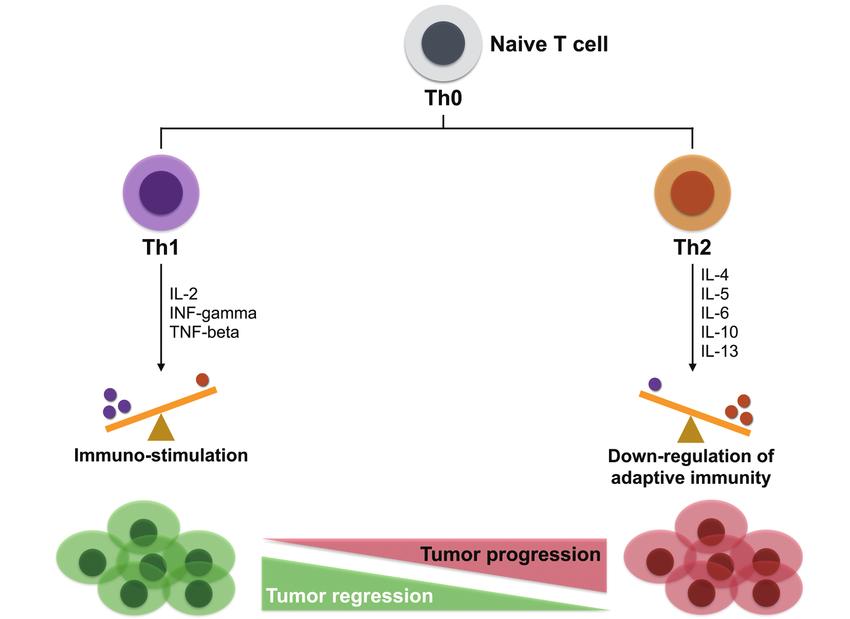 Effects of Th1/Th2 on tumor progression (Lin et al., 2017).
Effects of Th1/Th2 on tumor progression (Lin et al., 2017).
What is The Difference Between CD4 Th1 and Th2?
| Feature | Th1 Cells | Th2 Cells |
|---|---|---|
| Primary Function | Cellular immunity, defense against intracellular pathogens | Humoral immunity, defense against extracellular pathogens and allergens |
| Key Cytokines | IFN-γ, IL-2, TNF-α | IL-4, IL-5, IL-10, IL-13 |
| Transcription Factor | T-bet | GATA3 |
| Primary Stimuli | IL-12, IFN-γ | IL-4, thymic stromal lymphopoietin (TSLP) |
| Target Pathogens | Viruses, intracellular bacteria, protozoa | Helminths, allergens, extracellular bacteria |
| Mechanisms of Action | - Activates macrophages for enhanced killing (IFN-γ) - Stimulates CD8⁺ T cells (IL-2) - Promotes IgG2a/IgG3 production for opsonization |
- Activates eosinophils and mast cells (IL-5, IL-9) - Stimulates IgE class switching (IL-4) - Induces mucus production for pathogen clearance (IL-13) |
| Associated Immune Response | Type 1 (Cell-mediated immunity) | Type 2 (Humoral immunity) |
| Role in Disease | Autoimmune diseases (e.g., rheumatoid arthritis, multiple sclerosis, type 1 diabetes) | Allergic diseases (e.g., asthma, eczema, allergic rhinitis) |
| Regulatory Factors | Promoted by IL-12 and IFN-γ, inhibited by IL-4 | Promoted by IL-4, inhibited by IFN-γ |
Th1/Th2 Cytokine Detection Methods
Accurate quantification of Th1/Th2 cytokines is pivotal for dissecting immune polarization mechanisms, yet traditional methods often fail to address the complexity of dynamic, low-abundance signaling networks. This section evaluates evolving technologies through the lens of experimental rigor and reproducibility.
Limitations of Conventional Approaches
ELISA (Enzyme-Linked Immunosorbent Assay)
Matrix Interference: Serum albumin and heterophilic antibodies in culture media (e.g., RPMI-1640) induce false positives by cross-linking capture/detection antibodies. Studies show up to 30% overestimation of IL-4 in 10% FBS-containing samples.
Singleplex Bottleneck: Sequential detection of IFN-γ, IL-2, and TNF-α consumes 300+ μL sample volume, rendering time-course studies in primary T cell cultures (typically <50 μL supernatants) unfeasible.
Dynamic Range Compression: Commercial IL-5 ELISA kits (e.g., R&D Systems DY205) exhibit linearity only within 15.6–1,000 pg/mL, missing critical subthreshold signaling events (<10 pg/mL).
Flow Cytometric Bead Array (CBA)
Signal-to-Noise Decay: In low-cellularity samples (e.g., cerebrospinal fluid), PE fluorescence intensity drops below detection threshold for IL-13 (<2 pg/mL), necessitating sample pooling that masks donor variability.
Cross-Talk Artifacts: Simultaneous detection of IL-4 and GM-CSF suffers from spectral overlap (e.g., FITC/PE-Cy7 channels), requiring compensation matrices that reduce resolution by 40%.
Multiplex Detection Revolution with xMAP Technology
The Luminex xMAP platform overcomes these barriers through three engineering breakthroughs:
Magnetic Bead Encoding System
- Digital Barcoding: 100 distinct magnetic bead regions (MagPlex® microspheres) are impregnated with precise ratios of infrared fluorophores, enabling simultaneous measurement of 12 Th1/Th2 targets without cross-reactivity (Figure 4A).
- Surface Chemistry: Carboxylated beads allow covalent antibody conjugation via EDC/sulfo-NHS chemistry, achieving 98% coupling efficiency and 6-month stability at 4°C.
Services you may be interested in:
Signal Amplification Architecture
- Biotin-Streptavidin Cascade: Detection antibodies are biotinylated, followed by phycoerythrin (PE)-conjugated streptavidin, amplifying low-abundance signals (e.g., IL-10 at 0.5 pg/mL) 1,000-fold versus direct labeling.
- Laser Excitation Optimization: Dual-laser configuration (532 nm + 635 nm) eliminates autofluorescence in tissue lysates, improving signal purity by 75% compared to single-laser systems.
Extended Dynamic Range
- Auto-Scaling Algorithm: Integrated 8-point calibration curves (0.5–50,000 pg/mL) automatically adjust gain settings, enabling IL-2 (high pg/mL) and IL-13 (low pg/mL) co-detection in the same well.
- Intra-assay Precision: Coefficient of variation (CV) for IL-5 replicates is ≤5.2%, outperforming ELISA (CV 8–15%) and CBA (CV 12–20%).
Applications of Th1/Th2 Cytokine Research
Vaccine Development and Immune Response Evaluation
Luminex technology accelerates vaccine development by simultaneously detecting multiple Th1/Th2 cytokines:
Adjuvant Screening and Optimization:
In research on new adjuvants (such as TLR agonists and nanoparticles), Luminex can analyze the dynamic changes of IL-2, IFN-γ (Th1), and IL-4, IL-5 (Th2) simultaneously, determining the adjuvant's bias towards a specific immune response type. For example, aluminum adjuvants (Alum) tend to induce Th2 responses, while Poly(I:C) enhances Th1 responses.
In mRNA vaccine development (such as COVID-19 vaccines), the IFN-γ/IL-4 ratio is used to evaluate the vaccine-induced Th1/Th2 balance, optimizing antigen design to avoid excessive Th2 responses leading to antibody-dependent enhancement (ADE) risks.
Animal Model Immunomonitoring:
In preclinical vaccine trials, using minimal sample volumes (<50 μL serum), Luminex detects over 10 cytokines simultaneously to rapidly assess immune protection effects in mouse or non-human primate models.
Immunotherapy Mechanism Research
Luminex technology provides data support for understanding the mechanisms of immunomodulatory drugs and cell therapies:
CAR-T Cell Therapy:
Detecting the Th1/Th2 cytokine spectrum (such as IL-2, IFN-γ, IL-6) in patient serum after CAR-T cell infusion evaluates the risk of cytokine release syndrome (CRS). For example, significant increases in IL-6 and TNF-α are associated with severe CRS.
In co-culture experiments, analyzing IFN-γ and granzyme B levels secreted by CAR-T cells quantifies their cytotoxic efficiency against tumor cells.
Checkpoint Inhibitor Research:
Combining anti-PD-1 therapy with IL-2 therapy, Luminex monitors the synergistic effects of Th1 cytokines, revealing mechanisms of enhanced efficacy.
Studying the impact of suppressive factors like IL-10 and TGF-β on Th1/Th2 balance in the tumor microenvironment guides combination therapy strategies.
Th1/Th2 Dynamics in Disease Models
Luminex technology accurately captures cytokine temporal changes in infection, allergy, or autoimmune disease models:
Infection Immunology Research:
In viral infection models (such as influenza or HIV), analyzing the peak times of Th1 factors (IFN-γ, TNF-α) resolves the relationship between immune protection and immunopathology.
In parasitic infections (such as malaria), detecting IL-4 and IL-13 dynamics evaluates the role of Th2 responses in pathogen clearance.
Allergy Model Construction:
In dust mite or peanut protein-induced mouse allergy models, quantifying IL-5 and IL-13 levels correlates with eosinophil infiltration, validating the efficacy of novel anti-allergic drugs (such as IL-4Rα inhibitors).
Cell Interaction and Signaling Pathway Analysis
Combining in vitro co-culture or organoid models, Luminex technology reveals cell communication mechanisms:
Th1/Th2 Differentiation Regulation:
In DC-T cell co-culture systems, detecting IL-12 (from dendritic cells) inducing Th1 differentiation and IL-4 (from mast cells) driving Th2 differentiation.
Studying the suppressive effects of regulatory T cells (Tregs) secreting IL-10 and TGF-β on Th1/Th2 balance.
Cytokine Network Modeling:
Through multi-time point detection, constructing dynamic interaction networks of Th1/Th2 cytokines (such as IFN-γ inhibiting IL-4 expression) for computational biology simulations.
Biomarker Screening and Precision Medicine
Luminex technology's high-throughput capability supports large-scale biomarker discovery:
Tumor Immune Subtyping:
In melanoma or lung cancer patients' tissue microenvironments, detecting Th1/Th2 cytokine spectra (such as IFN-γ/IL-10 ratios) predicts immune therapy response rates.
Identifying Th2-type factors (such as IL-13) associated with immune therapy resistance, developing combination blockade strategies.
Autoimmune Disease Stratification:
Through multicenter cohort studies, screening for characteristic Th1/Th2 factor combinations (such as IL-17A+IFN-γ) in rheumatoid arthritis (RA) patients enables disease subtype classification.
Reference:
- Lin, Chang-Ni, Chih-Yen Chien, and Hui-Ching Chung. "Are friends or foes? New strategy for head and neck squamous cell carcinoma treatment via immune regulation." International Journal of Head and Neck Science 1.2 (2017): 105-113.

