Rat Cytokines and Their Significance
Cytokines, intricate signaling molecules produced by various immune cells, orchestrate the communication and coordination of immune responses within the body. These small proteins play a pivotal role in mediating diverse physiological processes, including immune regulation, inflammation, and cellular differentiation. By functioning as messengers, cytokines facilitate interactions between cells, allowing the immune system to respond effectively to challenges ranging from infections to tissue repair.
The study of rat cytokines holds profound relevance in unraveling the intricacies of human immune mechanisms. Rats, owing to their physiological and genetic similarity to humans, serve as invaluable models for investigating various aspects of immunology. While these models cannot fully replicate the human immune system, they offer critical insights into basic immune processes that can be extrapolated to human contexts. Consequently, rat cytokine research contributes substantially to the comprehension of immune responses, providing key insights into the underlying mechanisms of diseases.
In the realm of cytokine research, rat models emerge as indispensable tools for scientific inquiry. The similarities in cytokine production, receptor binding, and downstream signaling pathways between rats and humans facilitate the translation of findings from rat studies to potential clinical applications. This translational capacity makes rat cytokine research a cornerstone in the development of therapeutic interventions for human diseases, as it helps bridge the gap between preclinical studies and clinical trials.
Major Rat Cytokines and Their Roles in Diseases
Rat cytokines encompass a diverse array of molecules, including interleukins, interferons, tumor necrosis factors, and chemokines, each holding a distinctive role in shaping immune responses and influencing disease outcomes.
Interleukins (ILs): Interleukins are multifunctional cytokines that modulate interactions between immune cells, orchestrating their responses to challenges. For instance, IL-2 is critical for T-cell proliferation, bolstering immune reactions against infections and cancers. Conversely, dysregulation of IL-6 has been implicated in chronic inflammation and autoimmune disorders.
Interferons (IFNs): IFNs play a pivotal role in antiviral defense and immune regulation. IFN-γ, a prominent member, enhances macrophage activation and promotes cellular immunity, crucial for containing infections. However, an excess of IFN-γ has been linked to autoimmune disorders.
Tumor Necrosis Factors (TNFs): TNFs are key regulators of inflammation and immune homeostasis. TNF-α is a central mediator of inflammatory responses, inducing fever, cell apoptosis, and immune cell activation. Inappropriately elevated TNF-α levels contribute to chronic inflammatory diseases.
Chemokines: Chemokines guide immune cell migration to sites of infection or inflammation. CCL2, for instance, attracts monocytes to inflamed tissues, promoting wound healing but potentially leading to tissue damage if uncontrolled.
These cytokines collectively contribute to immune regulation, inflammation, and disease progression in a nuanced manner. Immune regulation involves the delicate balance between pro-inflammatory cytokines, which initiate immune responses, and anti-inflammatory cytokines that dampen them. This equilibrium is crucial for maintaining immune homeostasis, and disturbances can lead to immunopathologies.
Inflammation, initially a protective response against pathogens, can become detrimental if sustained. Pro-inflammatory cytokines trigger vasodilation, recruit immune cells, and amplify the immune response. While this is pivotal for clearing infections, chronic inflammation is implicated in various diseases, including autoimmune disorders, cardiovascular diseases, and neurodegenerative conditions.
Disease progression is intricately linked to cytokine dysregulation. Altered cytokine profiles can contribute to pathogenesis by promoting tissue damage, impeding proper immune responses, or fueling aberrant cell growth. By studying how specific cytokines drive disease processes in rat models, researchers gain insights into potential therapeutic targets for mitigating diseases and promoting overall health.
Select Service
Designing Cytokine Experiments: Guidelines and Best Practices
Designing cytokine experiments requires careful planning to ensure accurate and meaningful results. Proper experimental design, appropriate controls, optimal sample sizes, and robust statistical analysis are essential components of a successful study. Here are some guidelines and best practices to consider:
1. Define Clear Objectives:
Clearly outline the research questions or hypotheses you aim to address with your cytokine experiment. This will guide all subsequent steps of the experimental design.
2. Selecting Appropriate Controls:
Incorporate suitable controls to establish baseline cytokine levels and evaluate experimental effects. Controls may include:
Negative Controls: Samples without treatment or exposure, providing a baseline cytokine profile.
Positive Controls: Samples with known cytokine stimulants, validating the assay's sensitivity and accuracy.
Vehicle Controls: Samples treated with the vehicle used to administer the experimental agent, ensuring observed effects are not due to the vehicle itself.
3. Determining Sample Sizes:
Sample sizes should be statistically determined to ensure adequate power for detecting meaningful differences. Factors influencing sample size calculation include desired effect size, variability, significance level (alpha), and statistical power (1 - beta).
4. Randomization and Blinding:
Randomly assign samples to different treatment groups to minimize bias. Additionally, consider blinding experimenters to sample identities to prevent unintentional influence on data collection and analysis.
5. Experimental Replicates:
Repeat experiments with multiple independent samples (biological replicates) to account for biological variability. Technical replicates involve analyzing the same sample multiple times to assess assay precision.
6. Time Points and Dosing:
For time-course experiments or dose-response studies, carefully select time points and dosages that align with your research objectives. Ensure consistency in treatment administration.
7. Statistical Analysis:
Choose appropriate statistical methods to analyze cytokine data. Common approaches include t-tests, analysis of variance (ANOVA), and non-parametric tests. Consider correcting for multiple comparisons to control the risk of false positives.
8. Data Normalization:
Normalize cytokine data to account for differences in sample sizes, dilutions, or other factors that could affect absolute cytokine concentrations. Normalization enhances the comparability of data across samples.
9. Data Presentation:
Present cytokine data with descriptive statistics (mean, standard deviation) and graphical representations (bar graphs, heatmaps) to visualize trends and variations.
10. Data Validation:
Validate cytokine measurements using quality control measures, including standard curves, assay precision, and recovery rates. This ensures the accuracy and reliability of your data.
11. Reproducibility:
Document experimental procedures, conditions, and protocols meticulously to facilitate reproducibility. Transparent reporting of methods enhances the credibility of your findings.
Luminex Cytokine Analysis for Rat Models
Luminex technology revolutionizes cytokine analysis by enabling the simultaneous quantification of multiple cytokines in a single sample. This multiplexing capability eliminates the need for multiple individual assays, conserving precious samples and time. You can now obtain comprehensive cytokine profiles from a single experiment, providing a holistic view of immune responses in rat models.
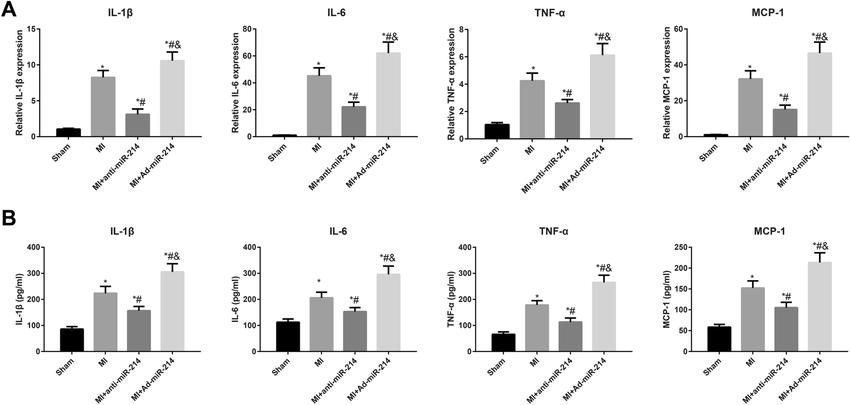 The
expression levels of the inflammatory cytokines in rats (Cheng et al., 2021).
The
expression levels of the inflammatory cytokines in rats (Cheng et al., 2021).
Applications of Luminex Cytokine Analysis in Rat Models
Disease Mechanisms: Luminex assays help unravel intricate cytokine networks associated with disease progression. Researchers can pinpoint dysregulated cytokine profiles that contribute to disease states, aiding in identifying potential therapeutic targets.
Drug Development: Luminex technology facilitates the assessment of cytokine responses to drug interventions. This insight is invaluable for evaluating treatment efficacy, optimizing dosages, and understanding the mechanisms of action.
Biomarker Discovery: The comprehensive cytokine data generated by Luminex assays hold promise for identifying novel biomarkers associated with diseases. These biomarkers can serve as diagnostic tools or indicators of treatment responses.
Reference:
- Cheng, Xiao-Jing, Lei Li, and Ben-Qiang Xin. "MiR-124 regulates the inflammation and apoptosis in myocardial infarction rats by targeting STAT3." Cardiovascular Toxicology 21.9 (2021): 710-720.