Defining the Roles in Immune Communication
The immune system is a complex network of cellular and molecular components that work together to defend the body from pathogens, maintain tissue homeostasis, and regulate immune responses. Among the critical signaling molecules involved in this system are cytokines and chemokines—two families of immune modulators with distinct but interrelated roles in cellular communication.
- Cytokines are a broad family of signaling molecules that regulate immune responses, cell proliferation, differentiation, and survival. These include interleukins (e.g., IL-2, IL-6), interferons (e.g., IFN-γ), and tumor necrosis factors (e.g., TNF-α). They are multifunctional and play a significant role in inflammatory responses, immune system activation, and tissue repair processes.
 Cytokine
Cytokine
- Chemokines are a subclass of cytokines with a specialized function: guiding the directed migration of cells, primarily immune cells, to specific sites of inflammation or injury. They include molecules such as CXCL12 and CCL5, which guide cellular movement through gradients, thereby coordinating immune cell trafficking and tissue localization.
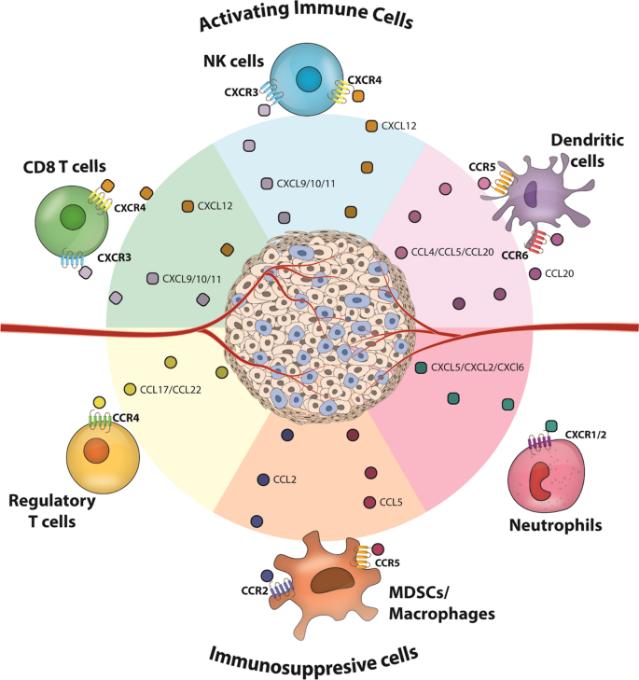 Key chemokines inducing immune infiltration into solid tumors (Kohli et al., 2022).
Key chemokines inducing immune infiltration into solid tumors (Kohli et al., 2022).
From an evolutionary standpoint, chemokines represent a branch of cytokine functionality that has preserved the chemotactic properties of early immune responses but evolved distinct structural and functional features tailored to cell migration.
Molecular Architecture: Structural Determinants of Function
The structures of cytokines and chemokines are crucial to their roles in immune response. Cytokines have diverse structures that support their broad functions, while chemokines share key structural elements tailored to cell migration.
Cytokines
Cytokines display a range of structural configurations, each linked to specific immune functions:
- α-Helical Bundles: For instance, IL-4 has an α-helical structure that enables binding to its receptor, driving immune responses like T-cell differentiation.
- β-Trefoil Structures: TGF-β adopts a β-trefoil structure, which is essential for regulating processes like immune suppression and tissue repair.
- Monomeric and Multimeric Forms: Some cytokines, such as TNF-α, form trimers, which enhance their ability to activate signaling pathways like NF-κB, influencing inflammation and cell death.
Chemokines
Chemokines have a more conserved structure focused on their chemotactic function:
- Four-Cysteine Motif: This motif, common across all chemokines, stabilizes their structure and enables effective binding to receptors. For example, CXCL12 uses its cysteine-rich region to interact with the CXCR4 receptor, guiding cell migration.
- N-terminal Domain: The N-terminal region of chemokines, rich in acidic residues, is critical for receptor interaction and activation. This is vital for chemokines like CXCL8 to recruit immune cells like neutrophils.
- Dimerization: Many chemokines, such as CXCL8, form dimers that increase receptor binding and signaling, amplifying their chemotactic response.
Genomic Organization
- Chemokine Clusters: Chemokine genes are often clustered on specific chromosomes (e.g., human chromosome 17), which allows for coordinated expression of related chemokines like CCL2 and CCL3.
- Cytokine Gene Distribution: Cytokines are dispersed across different chromosomes (e.g., IL-2 on chromosome 4), allowing independent regulation based on specific immune needs.
Functional Specialization: Mechanisms of Action
Chemokines: Masters of Cellular Navigation
Chemokines are specialized in guiding immune cells to specific locations, playing a key role in immune surveillance, inflammation, and tissue repair. Their primary function is to create chemotactic gradients, which direct cells to areas where they are needed most.
- Chemotaxis via GPCR Activation: Chemokines activate G-protein-coupled receptors (GPCRs) on immune cells, such as CXCR4 and CCR2, which initiate downstream signaling pathways. This leads to changes in cell shape and movement along the concentration gradient. For example, CXCL12 binds to CXCR4 to guide the migration of T-cells and hematopoietic stem cells to lymphoid tissues.
- Non-Chemotactic Functions: In addition to directing migration, chemokines also have non-chemotactic roles. CXCL8 (IL-8), for instance, promotes angiogenesis (blood vessel formation) and contributes to tissue repair by stimulating endothelial cell growth.
- Lymphocyte Homing: Chemokines also regulate the homing of lymphocytes to secondary lymphoid organs. CCL21 and CXCL13 guide B-cells and T-cells to lymph nodes, enhancing immune surveillance and activation.
Cytokines: Diverse Immune Modulators
Cytokines have a broader functional scope, with roles that extend beyond migration to immune modulation, cell differentiation, and regulation of inflammation.
- Inflammation Regulation: Cytokines such as IL-1β, TNF-α, and IL-6 promote inflammatory responses, helping to recruit immune cells and activate the immune system. Conversely, IL-10 and TGF-β suppress inflammation and promote tissue repair and immune tolerance.
- Cell Fate Determination: Cytokines also influence the differentiation of immune cells. For example, IL-12 promotes the differentiation of Th1 cells, which drive cellular immunity, while IL-4 favors Th2 differentiation, supporting humoral immunity. TGF-β directs the development of T-regulatory cells (Tregs), which maintain immune tolerance.
- Immune Modulation in cancer: Cytokines are involved in the immune response to tumors. IFN-γ enhances the ability of immune cells to recognize and kill cancer cells, while IL-10 and TGF-β are implicated in immune suppression within the tumor microenvironment.
Signaling Pathways
Both cytokines and chemokines initiate signaling pathways that result in immune responses, but the mechanisms and pathways differ.
- Chemokine Signaling: Chemokine receptors, as GPCRs, primarily utilize G-protein-dependent pathways (e.g., Ca²⁺ mobilization, MAPK activation) to trigger cell movement. In addition, some chemokines also use β-arrestin signaling, which can regulate receptor desensitization or promote cell survival.
- Cytokine Signaling: Cytokines typically signal through more complex pathways like JAK-STAT (e.g., IFN-γ), SMAD (e.g., TGF-β), and NF-κB (e.g., TNF-α, IL-1β) pathways. These pathways regulate immune responses, inflammation, cell survival, and apoptosis.
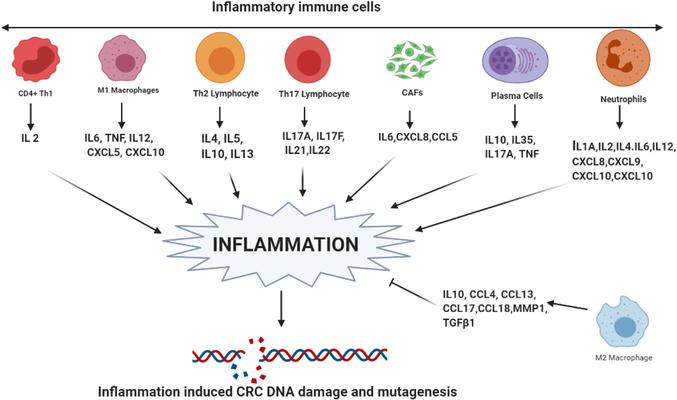 Cytokines and chemokines as inducers of inflammation (Bhat et al., 2022).
Cytokines and chemokines as inducers of inflammation (Bhat et al., 2022).
Receptor Systems and Specificity
Chemokine Receptors
Chemokine receptors are a subtype of G-protein coupled receptors (GPCRs) and play a crucial role in mediating the chemotactic effects of chemokines. These receptors have specific structural features that contribute to their ability to bind chemokines and initiate the necessary signaling events.
- Seven-Transmembrane Domain Structure: Chemokine receptors typically have a seven-transmembrane domain configuration, with their N-terminal regions located extracellularly and C-terminal regions inside the cell. The extracellular N-terminal region contains specific acidic residues that are important for binding to the chemokine's core structure.
- Receptor Binding Specificity: Each chemokine receptor is highly specific to its ligands. However, some chemokine receptors can bind to multiple chemokines, and some chemokines can interact with multiple receptors. For example, CCR5 binds to CCL3, CCL4, and CCL5, while CXCR4 binds to CXCL12 but can also interact with other chemokines in the CXCL family.
- Receptor Dimerization: Some chemokine receptors exhibit a tendency to dimerize upon activation. This can lead to enhanced receptor activation and signaling. This dimerization can be essential for the function of specific receptors such as CCR5, which is implicated in HIV infection.
- Chemokine Receptor Redundancy: There is significant redundancy within the chemokine receptor system. Multiple receptors can recognize similar ligands, and the same receptor can bind different chemokines. This redundancy enhances the immune system's ability to respond to diverse inflammatory signals and adjust to various pathologies.
Examples:
- CCR5: A receptor found on T-cells and macrophages, CCR5 binds to several CC chemokines, contributing to immune cell recruitment to sites of inflammation.
- CXCR4: CXCR4, primarily expressed on T-cells, hematopoietic cells, and endothelial cells, interacts with CXCL12 to guide cells to lymph nodes, bone marrow, and sites of injury.
Cytokine Receptors
Unlike chemokine receptors, cytokine receptors typically have a single transmembrane domain, but they often function as part of larger receptor complexes. The receptor structure is key to the specificity of cytokine signaling.
- Type I/Type II Cytokine Receptors: These receptors have a single transmembrane helix and are classified based on structural features. The Type I cytokine receptors (e.g., IL-2R) contain a common gamma-chain that forms part of the receptor complex. The Type II cytokine receptors (e.g., IFN-γR) share a conserved structure but activate different downstream signaling pathways.
- TNF Receptor Superfamily: Members of the TNF receptor superfamily (e.g., TNFR1) have a characteristic extracellular cysteine-rich domain and are involved in immune regulation and apoptosis. Upon ligand binding, these receptors form trimers that activate signaling pathways like NF-κB, leading to inflammation and cell death.
- Receptor Complex Formation: Many cytokines require receptor complexes to function. For example, the IL-6 receptor complex consists of the IL-6 receptor and gp130, which dimerize upon ligand binding to activate intracellular signaling cascades like JAK-STAT.
Examples:
- IL-2 Receptor: The IL-2 receptor (IL-2R) is a key cytokine receptor involved in the proliferation of T-cells. It exists as a heterotrimeric complex (α, β, γ chains) that is essential for signaling. Binding of IL-2 induces T-cell proliferation, essential for immune responses.
- TNF Receptor 1 (TNFR1): TNFR1 is a pivotal receptor in inflammation and cell survival. Upon TNF-α binding, it activates NF-κB signaling, promoting pro-inflammatory responses and apoptosis in some immune cells.
Cross-Talk and Redundancy
Both chemokine and cytokine receptor systems exhibit cross-talk and redundancy that enhance immune responsiveness and flexibility. Cross-talk refers to the ability of different receptors to influence each other's signaling, often leading to a synergistic immune response.
- Chemokine Receptor Cross-Talk: For instance, CXCR3, primarily involved in immune cell trafficking, can also influence the expression of genes induced by interferons, illustrating how chemokine receptors can influence cytokine signaling and immune cell function.
- Cytokine Receptor Redundancy: Cytokine receptors, too, demonstrate redundancy, where different cytokines can activate similar signaling pathways. For example, both IL-6 and IL-11 share the gp130 subunit and activate similar JAK-STAT signaling pathways, contributing to inflammation and tissue remodeling.
Table of Comparison Between Cytokines and Chemokines
| Feature | Cytokines | Chemokines |
|---|---|---|
| Definition | Small signaling proteins regulating immune responses, inflammation, and cell communication. | Subclass of cytokines specialized in directing cell migration via chemotaxis. |
| Structure | Diverse structures (proteins, glycoproteins); no conserved structural motifs. | Characterized by conserved cysteine motifs (e.g., CC, CXC, CX3C) forming disulfide bonds. |
| Primary Function | Regulate immune cell activation, differentiation, and inflammation; modulate cell growth and apoptosis. | Attract immune cells to sites of infection/injury; guide leukocyte trafficking. |
| Key Subtypes | Interleukins (IL-1, IL-6), interferons (IFN-γ), TNF-α, TGF-β. | Classified by cysteine motifs: CCL (e.g., CCL2, CCL5), CXCL (e.g., CXCL8, CXCL12). |
| Signaling Receptors | Bind to specific receptors (e.g., IL-6R, TNF-R); activate JAK-STAT, NF-κB pathways. | Bind to G-protein-coupled receptors (GPCRs) (e.g., CCR, CXCR). |
| Role in Inflammation | Pro-inflammatory (e.g., TNF-α, IL-1β) or anti-inflammatory (e.g., IL-10). | Recruit neutrophils, monocytes, and T-cells; amplify inflammatory responses. |
| Examples | IL-2 (T-cell activation), TNF-α (pro-inflammatory), IL-10 (anti-inflammatory). | CCL5/RANTES (monocyte chemotaxis), CXCL8/IL-8 (neutrophil recruitment). |
| Associated Diseases | Autoimmune diseases (e.g., rheumatoid arthritis), sepsis, cancer. | Chronic inflammation, HIV (via CCR5/CXCR4), metastasis (e.g., CXCR4 in breast cancer). |
Research Methodologies: Analyzing Chemokines and Cytokines
Multifactor Analysis Using Luminex xMAP Technology
Luminex xMAP technology is a bead-based multiplex assay system that enables the detection and quantification of multiple analytes (including cytokines and chemokines) in a single sample. This method is widely used in immunology and cellular biology to study the complex interplay between immune signaling molecules and their roles in immune responses.
Luminex xMAP Technology: Key Features
- Multiplexing Capability: Luminex xMAP technology utilizes color-coded beads that are coated with capture antibodies specific to different cytokines or chemokines. Each bead set corresponds to a different analyte, allowing researchers to measure several cytokines and chemokines in a single sample simultaneously. This is especially useful in profiling the immune environment in disease conditions like inflammation, infection, or cancer.
- High Sensitivity and Dynamic Range: The system offers high sensitivity and dynamic range, enabling the detection of cytokines at low concentrations in biological samples such as plasma, serum, or tissue homogenates. This is particularly important for studying low-abundance cytokines and chemokines that play crucial roles in immune regulation.
- Quantification and Profiling: Luminex xMAP technology can quantitatively measure the concentrations of cytokines and chemokines in a given sample, providing a comprehensive profile of immune system activity. By analyzing multiple analytes in parallel, this method helps to understand the complex immune responses and cytokine networks that drive disease progression.
Services you may be interested in:
Applications in Cytokine and Chemokine Research
Inflammatory Diseases: In conditions like rheumatoid arthritis, Crohn's disease, or asthma, Luminex xMAP technology can be used to profile pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and chemokines (e.g., CCL2, CXCL8) to understand the immune responses that contribute to tissue damage and disease exacerbation.
Cancer Immunology: The technology is also applied in cancer immunology to profile the immune microenvironment. By measuring immune checkpoint regulators (e.g., PD-L1, CTLA-4) and cytokines involved in tumor progression, such as IL-10 and TGF-β, Luminex can help identify potential therapeutic targets or biomarkers for immunotherapy.
Viral and Bacterial Infections: In infectious disease research, Luminex xMAP technology helps assess the levels of pro-inflammatory cytokines and chemokines during infection, providing insights into the host immune response and the role of immune mediators in controlling pathogen spread. It can also help to identify immune dysregulation in chronic infections, such as HIV or tuberculosis.
Luminex vs. Traditional ELISA
While traditional Enzyme-Linked Immunosorbent Assays (ELISA) are commonly used to quantify single analytes, Luminex xMAP technology offers a clear advantage by allowing the analysis of multiple cytokines and chemokines in a single well. This not only reduces sample volume but also enhances the efficiency and throughput of the study. Furthermore, Luminex provides superior quantitative data, making it easier to assess subtle differences in cytokine and chemokine levels between different experimental conditions or patient groups.
Omics Approaches: Complementing Luminex with Proteomics
Luminex technology can be used in combination with proteomic approaches, such as mass spectrometry, to provide even more detailed insights into cytokine and chemokine networks. For example, cytokine profiles from Luminex can be integrated with proteomic data to understand the dynamic regulation of immune responses across different tissues or during various disease states.
By combining Luminex xMAP assays with quantitative proteomics, researchers can study how changes in cytokine and chemokine networks influence the progression of diseases like cancer, autoimmune disorders, and chronic infections.
References:
- Kohli, Karan, Venu G. Pillarisetty, and Teresa S. Kim. "Key chemokines direct migration of immune cells in solid tumors." Cancer gene therapy 29.1 (2022): 10-21.
- Bhat, Ajaz A., et al. "Cytokine‐and chemokine‐induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy." Cancer Communications 42.8 (2022): 689-715.
- Cameron, Mark J., and David J. Kelvin. "Cytokines, chemokines and their receptors." Madame curie bioscience database [Internet]. Landes Bioscience, 2013.

