What are Cytokine Storms?
Cytokine storms, also known as cytokine release syndrome (CRS), are a severe immune reaction characterized by an uncontrolled release of pro-inflammatory cytokines. These storms can occur in response to a variety of triggers, including infections, immune therapies, and certain cancers. Understanding the pathophysiology of cytokine storms is paramount, as they can lead to widespread tissue damage, organ failure, and even death. In recent years, the heightened awareness of cytokine storms due to the COVID-19 pandemic has catalyzed significant advancements in both research and clinical management.
This article aims to delve into the molecular mechanisms of cytokine storms, identify the key cytokines involved, and explore the latest analytical techniques for profiling these immune responses. Additionally, we will explore the intersection of cytokine storms with disease processes and the critical role of animal models and genetic factors in furthering our understanding of this phenomenon.
Causes and Mechanisms of Cytokine Storms
Cytokine storms occur when the immune system's response is dysregulated, often in the face of an infection or therapeutic intervention. The rapid and excessive release of cytokines, such as IL-6, TNF-alpha, and IL-1, overwhelms the immune system's capacity for regulation, leading to systemic inflammation, endothelial dysfunction, and tissue damage.
Pathophysiological Basis
The root cause of cytokine storms is typically an exaggerated immune response. In a normal immune response, the immune system detects pathogens or injuries and mounts an inflammatory response to fight the infection or repair tissue damage. However, in the case of a cytokine storm, this response becomes disproportionate, often leading to widespread tissue damage and systemic inflammation.
This overactive immune reaction is frequently observed in infections like influenza and COVID-19, as well as in cancer immunotherapies, such as CAR T-cell therapy. Cytokines like IL-6, TNF-alpha, and IL-1 are key players in this process, driving inflammation and attracting immune cells to the site of infection or damage.
Cellular Players
At the heart of cytokine storms are various immune cells, including macrophages, T-cells, and natural killer (NK) cells. These cells, when hyperactivated, can release significant amounts of cytokines, leading to what is often referred to as a "cytokine cascade." Macrophages, in particular, play a central role in initiating and amplifying this process by releasing pro-inflammatory cytokines that, in turn, stimulate further immune cell activation.
In addition to macrophages, T-cells and NK cells contribute to cytokine storm responses. While these cells are crucial in combating pathogens, their excessive activation during a cytokine storm leads to severe tissue damage and exacerbates inflammation.
Molecular Pathways
Several molecular pathways contribute to the regulation and amplification of cytokine release. Pattern recognition receptors (PRRs) on immune cells detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), triggering the release of cytokines. For example, TLRs (Toll-like receptors) play a critical role in recognizing foreign invaders and initiating the immune response. However, when PRRs are hyperactivated, it leads to an exaggerated immune response that causes excessive cytokine production.
The activation of the NF-κB pathway is central to cytokine production. NF-κB regulates the transcription of many pro-inflammatory cytokines and is often upregulated in severe immune responses. The JAK-STAT signaling pathway, particularly activated by IL-6, also plays a significant role in the propagation of cytokine storms. Additionally, the MAPK signaling pathway contributes to the regulation of inflammatory cytokine gene expression, while inflammasomes, such as NLRP3, are involved in the production of IL-1β and subsequent pyroptosis, a form of cell death that can exacerbate inflammation.
Key Cytokines Involved in Cytokine Storms
| Cytokine | Role in Cytokine Storm | Key Effects |
|---|---|---|
| IL-6 | A central mediator in cytokine storms, produced by various immune cells like macrophages. | Stimulates acute-phase responses, induces fever, and contributes to tissue damage. Plays a major role in the progression of diseases like COVID-19 and autoimmune disorders. |
| TNF-alpha | A potent pro-inflammatory cytokine produced by macrophages and other immune cells. | Increases vascular permeability, induces apoptosis, promotes inflammation, and contributes to systemic shock. Essential in acute inflammatory responses. |
| IL-1 | A key cytokine in initiating and amplifying inflammation, released by macrophages and dendritic cells. | Causes fever (pyrogenic effect), promotes inflammation, leads to tissue damage, and is involved in systemic inflammatory response syndrome (SIRS). |
| IL-8 | A chemokine that recruits neutrophils to sites of infection and inflammation. | Induces neutrophil chemotaxis, contributes to tissue damage, and enhances inflammation. Important in bacterial and viral infections. |
| IL-18 | An inflammasome-activated cytokine that amplifies the immune response. | Induces IFN-gamma production, stimulates NK cells, enhances Th1 cell responses, and contributes to systemic inflammation and tissue damage. |
| IL-10 | An anti-inflammatory cytokine that aims to control excessive immune responses. | Inhibits the production of pro-inflammatory cytokines but often insufficient during cytokine storms, potentially allowing the storm to escalate. |
| TGF-beta | A cytokine that regulates immune tolerance and suppresses inflammation under normal conditions. | Can limit excessive immune responses but is often overridden in the context of cytokine storms, leading to immune dysregulation and fibrosis in some tissues. |
| IFN-gamma | A cytokine primarily involved in the activation of macrophages and regulation of immune responses. | Promotes inflammation, enhances antigen presentation, and can contribute to tissue damage during a storm, particularly in viral infections. |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor stimulates the production and activation of macrophages and neutrophils. | Amplifies the inflammatory cascade, contributes to the expansion of inflammatory cell populations, and is implicated in severe inflammation and immune dysregulation. |
| MCP-1 (CCL2) | A chemokine that recruits monocytes to inflamed tissues. | Promotes the recruitment of monocytes, leading to tissue infiltration and exacerbating inflammation during cytokine storms. |
Molecular Signaling Pathways in Cytokine Storms
The molecular signaling pathways that drive cytokine storms are complex and interrelated, involving multiple key players that either amplify or attenuate immune responses.
NF-κB Pathway
The NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway is a central regulator of immune responses and inflammation. This pathway is activated by the recognition of PAMPs or DAMPs through PRRs. Once activated, NF-κB induces the transcription of pro-inflammatory cytokines, including IL-6, TNF-alpha, and IL-1beta, leading to a cascade of immune responses. NF-κB's involvement in cytokine storms makes it an attractive target for drug development, as inhibiting this pathway could reduce the severity of cytokine storms in both viral infections and cancer immunotherapy-related CRS.
JAK-STAT Signaling
The JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway plays a crucial role in mediating the effects of cytokines like IL-6 and interferons. When activated, JAK-STAT signaling induces the transcription of various genes responsible for immune cell survival, differentiation, and proliferation. In cytokine storms, this pathway becomes hyperactivated, amplifying the immune response and contributing to the prolonged inflammation seen in conditions like COVID-19 and CAR T-cell therapy. Targeting the JAK-STAT pathway may hold promise for controlling excessive immune responses in these settings.
Inflammasomes and IL-1 Signaling
The inflammasome is a complex of proteins that assembles in response to cellular damage and infection. Upon activation, inflammasomes lead to the processing and release of IL-1beta, a key cytokine in cytokine storms. IL-1beta contributes to the amplification of the inflammatory response and is implicated in several diseases, including autoimmune disorders and viral infections. Targeting inflammasome activation could offer a novel therapeutic strategy for controlling cytokine storm-driven diseases.
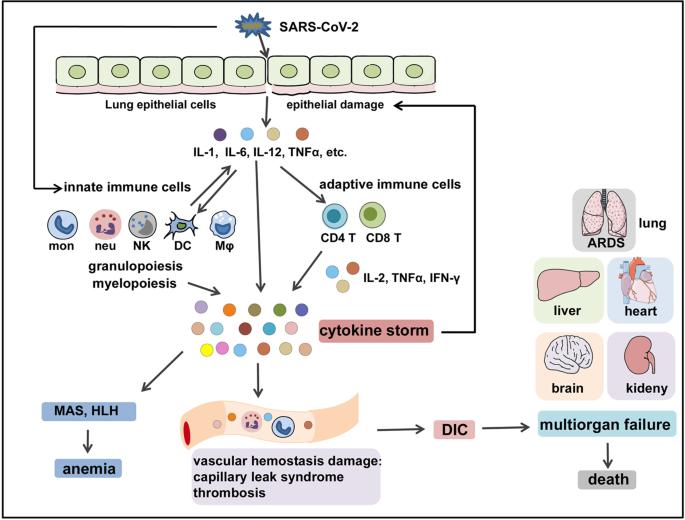 The immunopathological mechanisms of COVID-CS (Yang, Lan, et al., 2021).
The immunopathological mechanisms of COVID-CS (Yang, Lan, et al., 2021).
Crosstalk Between Cytokines and Other Biological Systems
Cytokine storms do not just affect the immune system. They also have far-reaching effects on other biological systems, leading to widespread damage.
Neuroinflammation
Cytokines such as IL-6 and TNF-alpha can cross the blood-brain barrier and activate glial cells in the brain, leading to neuroinflammation. This can contribute to symptoms like confusion, delirium, and fatigue, often seen in patients with cytokine storms.
Coagulation System
Excessive cytokine production leads to changes in the coagulation system, resulting in hypercoagulability. This is associated with an increased risk of thrombotic events, such as deep vein thrombosis (DVT) or pulmonary embolism, in patients suffering from cytokine storms.
Metabolic Reprogramming
Cytokine storms induce metabolic reprogramming in immune cells, enhancing glycolysis and altering mitochondrial function. This provides energy for the excessive immune response but also contributes to tissue damage.
Cytokine Profiling and Analysis Techniques
In the realm of immune pathology research, dynamically monitoring cytokine storms—a molecular "hurricane"—poses significant challenges. Traditional single-analyte assays (e.g., ELISA) struggle to unravel the synergistic network of mediators like IL-6, TNF-α, and IFN-γ due to limited throughput and high sample consumption. Luminex xMAP technology, with its high multiplexing capability and sensitivity, emerges as a pivotal tool for decoding this complexity.
Technological Innovation: From Single-plex to Panoramic Profiling
The core of Luminex technology lies in its fluorescent-coded microsphere system:
- Multiplex Detection: Utilizing dual-color (red/infrared) fluorescence encoding, a single experiment can simultaneously quantify up to 100 biomarkers. For instance, in COVID-19 preclinical models, the co-elevation of IL-6 and D-dimer has been linked to multi-organ failure risks.
- Ultra-High Sensitivity: With a detection limit as low as 0.1 pg/mL, the platform identifies low-abundance regulators (e.g., IL-1RA), enabling early warning of immune dysregulation.
- Sample Efficiency: Only 25 μL of serum, plasma, or cell supernatant is required, making it ideal for precious preclinical samples (e.g., murine models or CAR-T cell therapy studies).
Applications: From Mechanistic Insights to Preclinical Translation
Viral-Induced Cytokine Storms
In SARS-CoV-2-infected animal models, Luminex reveals that IL-6 and ferritin synergistically drive disease severity, while early fluctuations in IL-12p70 signal immune imbalance.
Influenza studies using a 46-plex panel uncover delayed type I interferon release correlated with epithelial damage, guiding antiviral drug development.
Immunotherapy Mechanism Exploration
For CAR-T therapy research, dynamic monitoring of IL-6 and IFN-γ "dual peaks" optimizes intervention timing, effectively reducing the risk of cytokine release syndrome (CRS).
In autoimmune disease models (e.g., macrophage activation syndrome), custom panels (including IL-18, CXCL9) quantify inflammasome activation, distinguishing primary from secondary hyperinflammation.
Multi-Omics Integration
Combined with metabolomics or single-cell sequencing, Luminex data elucidates cross-talk between lactate accumulation and T-cell exhaustion, supporting microenvironmental studies.
Tailored Detection Services
To address the complexity of cytokine storm studies, Creative Proteomics offers customized Luminex-based solutions with the following advantages:
1. Flexible Panel Design
- Preconfigured Panels: Cover core cytokines (e.g., IL-6, TNF-α, IFN-γ) for viral infection, cancer immunotherapy, or autoimmune research.
- Custom Assays: Add or remove targets (e.g., IFN-α/β, CXCL10) based on disease models (e.g., COVID-19, CAR-T therapy) or pathway-specific needs.
2. High Sensitivity and Sample Conservation
- Achieve 0.1 pg/mL sensitivity with minimal sample input (15 μL serum or cell supernatant), ideal for murine studies or in vitro co-culture systems.
3. Advanced Data Analysis
- Visualize cytokine clusters via heatmaps or radar plots to identify key drivers (e.g., IL-6-mortality correlation).
- Apply machine learning to derive predictive biomarker combinations (e.g., IL-6 >40 pg/mL + ferritin >1000 ng/mL for severe outcome risks).
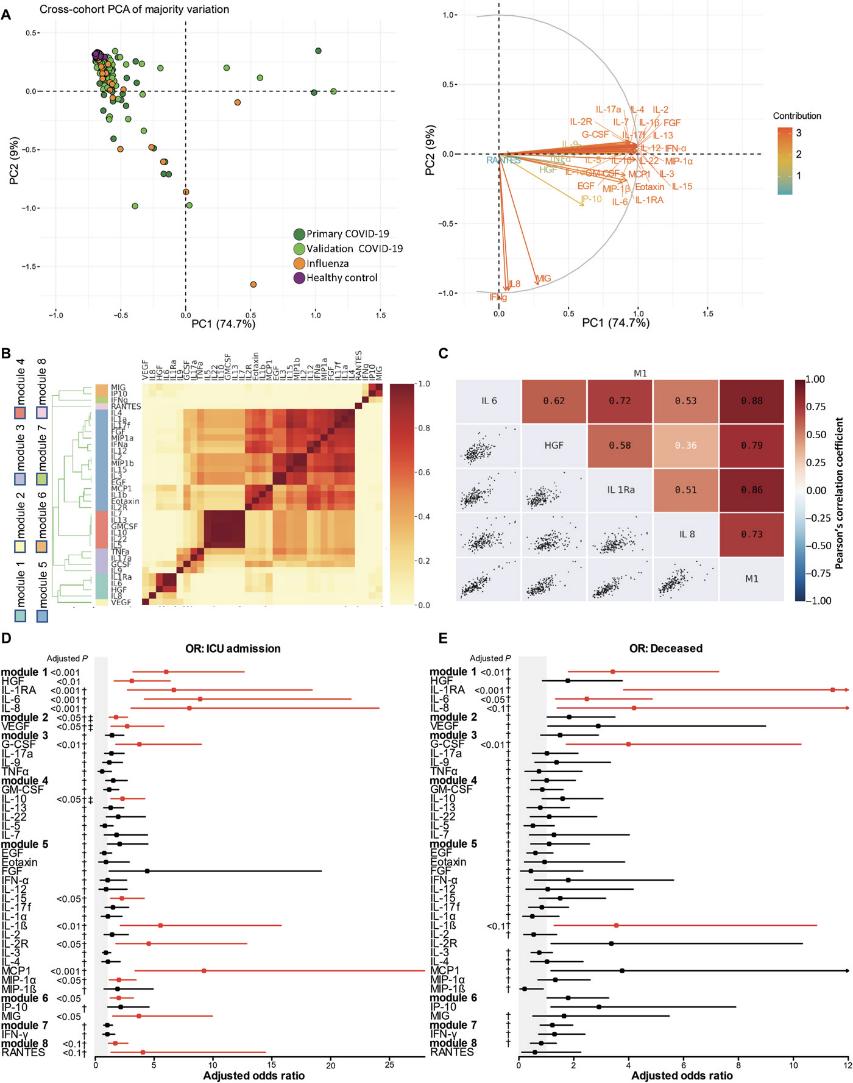 Cross-cohort comparisons. (A) PCA of 35 cytokines with loadings showing their effects. (B) Cytokine correlations from hierarchical clustering. (C) Module 1 cytokine correlations. (D, E) Forest plots of cytokine associations with ICU admission and death.
Cross-cohort comparisons. (A) PCA of 35 cytokines with loadings showing their effects. (B) Cytokine correlations from hierarchical clustering. (C) Module 1 cytokine correlations. (D, E) Forest plots of cytokine associations with ICU admission and death.
Service Scope Declaration
Our cytokine detection services are exclusively for basic scientific research and preclinical applications, including:
- Viral and Immune Mechanism Studies (e.g., inflammatory network dynamics in infection models)
- Drug Development and Safety Evaluation (e.g., CRS risk assessment in CAR-T therapy)
- Disease Model Validation (e.g., inflammasome activation in autoimmune disorders)
Services you may be interested in:
Cytokine Storms in Different Diseases
Cytokine storms are a pathological phenomenon seen across various disease states, and their manifestations and severity can differ based on the underlying condition.
Viral Infections
Viral infections, particularly those caused by highly pathogenic viruses like influenza, SARS-CoV-2 (COVID-19), and others, are strongly associated with the induction of cytokine storms. The severe inflammatory response observed in diseases like COVID-19 is characterized by the rapid, unregulated release of cytokines such as IL-6, TNF-alpha, and IL-1, leading to acute respiratory distress syndrome (ARDS) and multi-organ failure. In the case of SARS-CoV-2, the virus itself directly stimulates the immune system through toll-like receptors (TLRs), activating macrophages and dendritic cells to produce a wide array of pro-inflammatory cytokines. This cytokine release not only disrupts normal immune homeostasis but also worsens the infection's severity, leading to potentially fatal consequences.
In the context of viral infections, the cytokine storm can be considered a double-edged sword; while cytokines are essential for controlling and eliminating pathogens, their excessive release during an uncontrolled immune response contributes significantly to tissue damage and organ dysfunction.
Autoimmune Diseases
In autoimmune diseases, the immune system mistakenly targets the body's own cells, causing chronic inflammation. Diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) often present with persistent elevations in cytokines such as IL-6, TNF-alpha, and IL-1. In SLE, for instance, autoantibodies and immune complexes can activate the complement system and innate immune cells, triggering a cascade of inflammatory cytokine production. While cytokine storms are not always present in autoimmune conditions, their exacerbation can lead to disease flares and complications, contributing to the progression of tissue damage in organs like the kidneys, heart, and lungs.
The key difference in autoimmune-related cytokine storms is that these responses are often prolonged and more insidious compared to the acute storms seen in viral infections. Chronic inflammation from cytokine dysregulation in autoimmune diseases can drive progressive tissue destruction, which makes these conditions challenging to treat.
Cancer and Immunotherapies
One of the more alarming recent developments in cytokine storm research has been its association with cancer immunotherapies, particularly chimeric antigen receptor (CAR) T-cell therapy. CAR T-cell therapies, which engineer a patient's T cells to target specific cancer cells, have revolutionized cancer treatment, but they also pose significant risks. When CAR T-cells engage with their target cancer cells, they can rapidly release massive quantities of cytokines, causing Cytokine Release Syndrome (CRS). CRS is characterized by fever, hypotension, multi-organ dysfunction, and potentially fatal outcomes if not properly managed.
In these therapies, cytokines like IL-6, IL-1, and TNF-alpha can become markedly elevated, leading to life-threatening inflammation. While CRS is a well-known side effect of CAR T-cell therapy, its occurrence highlights the delicate balance needed in immune-modulating therapies. Research is now focused on strategies to minimize the severity of cytokine storms in these therapies, including the use of IL-6 inhibitors and other targeted treatments.
References:
- Yang, Lan, et al. "The signal pathways and treatment of cytokine storm in COVID-19." Signal transduction and targeted therapy 6.1 (2021): 255. https://doi.org/10.1038/s41392-021-00679-0
- Mudd, Philip A., et al. "Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm." Science advances 6.50 (2020): eabe3024. https://doi.org/10.1126/sciadv.abe3024
- Kessel, Christoph, et al. "Definition and validation of serum biomarkers for optimal differentiation of hyperferritinaemic cytokine storm conditions in children: a retrospective cohort study." The Lancet Rheumatology 3.8 (2021): e563-e573. https://doi.org/10.1016/S2665-9913(21)00115-6

