Allergic asthma is a chronic inflammatory airway disorder driven by dysregulated immune responses to environmental allergens. It is characterized by airway hyperresponsiveness, mucus hypersecretion, and variable airflow obstruction.
While symptoms such as coughing, wheezing, and breathlessness are visible indicators, the underlying molecular mechanisms are deeply rooted in immune system miscommunication, particularly through cytokine signaling networks.
Cytokines — small proteins secreted by immune and structural cells — coordinate inflammation, tissue remodeling, and immune activation. In allergic asthma, imbalances in cytokine profiles not only initiate but also perpetuate disease progression. Consequently, studying these complex cytokine networks is pivotal for understanding asthma pathogenesis, identifying biomarkers, and developing targeted therapies.
Traditional single-plex methods like ELISA, although reliable, cannot meet the demands for comprehensive cytokine profiling. As asthma involves multiple cytokines acting simultaneously, researchers require technologies capable of high-throughput, multiplexed detection. This gap is perfectly filled by Luminex multiplex cytokine detection technology, which revolutionizes the field by enabling simultaneous quantification of numerous cytokines with minimal sample input.
✅ Explore advanced multiplex solutions through Creative Proteomics' Luminex Cytokine Detection Service and accelerate your asthma research.
Cytokine Networks and Immune Cells in Allergic Asthma
The inflammatory process of allergic asthma is driven by a highly interconnected and dynamic cytokine network, involving both structural cells and immune cells. Each cytokine not only has distinct biological functions but also interacts with others to shape the inflammatory environment in a time- and space-dependent manner.
Airway Epithelial Cell Alarmins: Initiators of Inflammation
Airway epithelial cells serve as the first line of defense against inhaled allergens, pollutants, and pathogens. Upon sensing danger signals, epithelial cells rapidly release alarmin cytokines:
- Interleukin-25 (IL-25): Stimulates Th2 responses by enhancing IL-4, IL-5, and IL-13 production.
- Interleukin-33 (IL-33): Activates ILC2s, Th2 cells, mast cells, and basophils, contributing to airway hyperresponsiveness.
- Thymic Stromal Lymphopoietin (TSLP): Potentiates dendritic cells to drive naïve T cells toward Th2 polarization.
Key points to highlight:
- These alarmins act upstream of adaptive immunity, influencing early immune priming.
- They bridge innate and adaptive immune responses, ensuring a rapid escalation of inflammation.
- High levels of epithelial alarmins correlate with severe, steroid-resistant asthma.
Functional Specialization of Th2 Cell-Dominant Cytokines
T helper type 2 (Th2) cells are central architects of type 2 airway inflammation. Once activated by dendritic cells, Th2 cells secrete a panel of cytokines with distinct immunopathologic roles:
IL-4:
- Essential for B-cell class switching to IgE.
- IgE binds to FcεRI receptors on mast cells and basophils, sensitizing them for allergen-induced degranulation.
IL-5:
- Acts directly on eosinophils to promote differentiation, activation, and survival.
- Eosinophil infiltration into the airways leads to tissue damage, airway remodeling, and persistent inflammation.
IL-13:
- Drives goblet cell metaplasia and excessive mucus production.
- Contributes to airway fibrosis and increased bronchial hyperresponsiveness, a hallmark of severe asthma.
IL-9:
- Amplifies mast cell numbers in the airways.
- Enhances IL-13 production and mucous hyperplasia, further perpetuating allergic inflammation.
Synergistic amplification among these cytokines ensures redundancy, making the inflammatory process robust and difficult to disrupt with a single-targeted approach. Clinical trials blocking IL-4/IL-13 signaling have demonstrated success in reducing asthma exacerbations, validating the central role of Th2 cytokines.
 Resolution of allergic asthma. Allergic asthma exacerbations are associated with increased accumulation of Th2 in the airways which release TH2 (Th2) cytokines like IL-5, IL-9, and IL-13 (Finotto, Susetta, 2019).
Resolution of allergic asthma. Allergic asthma exacerbations are associated with increased accumulation of Th2 in the airways which release TH2 (Th2) cytokines like IL-5, IL-9, and IL-13 (Finotto, Susetta, 2019).
Synergistic Roles of Other Immune Cells and Cytokines
Beyond Th2 lymphocytes, other innate and adaptive immune cells critically shape the airway microenvironment:
Type 2 Innate Lymphoid Cells (ILC2s):
- Rapidly produce IL-5 and IL-13 upon stimulation by epithelial-derived IL-25, IL-33, and TSLP.
- Function independently of antigen presentation, enabling early-phase cytokine bursts even before T-cell activation.
Mast Cells:
- Key effector cells in immediate hypersensitivity responses.
- Release preformed mediators like histamine and newly synthesized cytokines (e.g., IL-4, IL-5) upon allergen crosslinking of IgE.
Eosinophils:
- Terminal effector cells recruited by IL-5.
- Release toxic granules (e.g., major basic protein, eosinophil cationic protein) that directly damage airway epithelium and stimulate further inflammation.
Basophils and B cells also contribute by producing IL-4, sustaining IgE production, and reinforcing Th2 immunity. Once initiated, these cells and their cytokines establish positive feedback loops that exacerbate and sustain airway inflammation, even in the absence of continuous allergen exposure.
Principles and Advantages of Luminex Multiplex Detection Technology
Luminex multiplex technology offers a powerful solution, combining high-throughput capability with superior sensitivity and specificity, surpassing many traditional immunoassay methods.
Multiplexing Principle of Luminex Technology and Comparison with ELISA
Luminex xMAP technology utilizes color-coded microspheres (beads), each internally dyed with a unique combination of fluorescent dyes to create a distinct spectral signature. Each bead is conjugated with a specific capture antibody targeting a particular analyte (e.g., IL-4, IL-5, TSLP).
Workflow overview:
- Bead sets are mixed and incubated with a biological sample containing multiple target cytokines.
- After washing, detection antibodies (biotinylated) specific to each cytokine are added.
- Streptavidin-phycoerythrin (SA-PE) is then introduced, which binds to biotin, producing a fluorescent signal proportional to the amount of cytokine bound.
- The beads are passed through a dual-laser flow cytometer, where one laser identifies the bead type (analyte) and another quantifies the PE signal (amount).
Comparison with traditional ELISA:
| Feature | Luminex | ELISA |
|---|---|---|
| Multiplex capability | 50-500 analytes per sample | 1 analyte per sample |
| Sample volume requirement | ~25-50 μL | 100-200 μL |
| Detection sensitivity | pg/mL to low fg/mL | typically pg/mL |
| Throughput | High (96/384-well plates) | Moderate |
| Dynamic range | Wide (3-5 logs) | Limited (2-3 logs) |
Key takeaway: Luminex assays reduce time, sample volume, and cost while providing richer, multidimensional data compared to conventional ELISAs.
✅ Interested in exploring this technology for your research? Visit our Luminex Cytokine Detection Service for customized assay solutions.
Technical Advantages: High Throughput, Sensitivity, and Sample Conservation
High Throughput
- Simultaneous quantification of up to 500 cytokines, chemokines, or growth factors per sample.
- Ideal for large-scale studies, e.g., asthma cohort profiling, biomarker discovery.
Exceptional Sensitivity and Dynamic Range
- Detects cytokine concentrations as low as sub-picogram levels.
- Provides broad dynamic ranges (3–5 logs), minimizing the need for multiple sample dilutions.
Minimal Sample Volume
- Requires as little as 25-50 μL of serum, plasma, bronchoalveolar lavage fluid (BALF), or cell culture supernatant.
- Critical advantage when sample availability is limited, such as in pediatric studies or animal models.
Reproducibility and Precision
- Standardized bead manufacturing ensures low inter-assay variability.
- Multiplex design inherently controls for technical variability, enhancing data reliability.
Applicable Cytokine Panels for Asthma Research
Luminex technology is particularly suited for analyzing asthma-associated cytokines, offering comprehensive panels that may include:
- Th2 cytokines: IL-4, IL-5, IL-13, IL-9
- Alarmins: TSLP, IL-25, IL-33
- Th17 cytokines: IL-17A, IL-17F
- Th1 cytokines: IFN-γ, IL-12
- Other inflammatory mediators: TNF-α, IL-6, GM-CSF
Customizable panels allow researchers to tailor the assay to their specific project needs, whether focusing on early inflammatory events, chronic airway remodeling, or drug response evaluation.
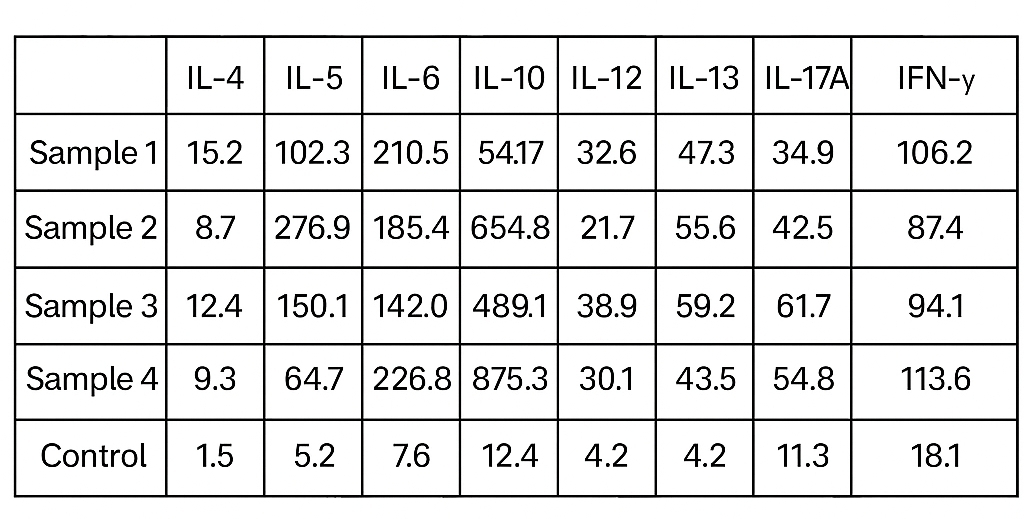 Example of Cytokine Panel Report
Example of Cytokine Panel Report
Application in Basic and Translational Asthma Research
Quantitative Analysis of Inflammatory Microenvironment in Animal Models and Cell Experiments
Animal models (e.g., ovalbumin-induced mouse models, house dust mite exposure models) and in vitro cell culture systems are widely used to dissect asthma pathogenesis.
Accurate cytokine profiling from these models helps:
- Evaluate disease progression.
- Understand immune cell interactions.
- Identify early biomarkers of airway inflammation.
How Luminex contributes:
- Simultaneous measurement of key cytokines (e.g., IL-4, IL-5, IL-13, TSLP, IL-17) from small-volume bronchoalveolar lavage fluid (BALF) or culture supernatants.
- Detects subtle shifts in cytokine milieu under experimental interventions (e.g., allergen sensitization, corticosteroid treatment).
This multiplexed, quantitative approach accelerates hypothesis testing and deepens mechanistic insights, ensuring data richness and reproducibility.
Association Studies Between Asthma Phenotypes and Cytokine Profiles
Asthma is increasingly recognized as a syndrome with multiple distinct endotypes, driven by different underlying immune mechanisms.
Common inflammatory phenotypes include:
- Eosinophilic asthma (high Th2 cytokines: IL-4, IL-5, IL-13)
- Neutrophilic asthma (high Th17 cytokines: IL-17A, IL-8)
- Mixed granulocytic asthma
- Paucigranulocytic asthma (low inflammatory signatures)
By applying Luminex panels:
- Researchers can cluster patients based on their cytokine signatures.
- Associate cytokine patterns with clinical parameters such as lung function (FEV1), exacerbation frequency, and response to biologics.
- Identify novel asthma endotypes, paving the way for precision medicine.
For further reading, see European Respiratory Society's guide on asthma phenotypes.
Dynamic Cytokine Monitoring in New Drug Target Screening and Pharmacodynamic Studies
In drug development pipelines for asthma, monitoring cytokine responses is critical for:
- Screening potential therapeutic targets.
- Evaluating pharmacodynamic effects of candidate drugs.
- Predicting efficacy and optimizing dosing regimens.
Using Luminex assays:
- Dynamic changes in a panel of cytokines can be monitored over time after drug administration.
- Cytokine suppression profiles (e.g., decreased IL-5, IL-13, or TSLP) offer early proof-of-concept for drug activity.
- Rapid feedback helps prioritize the most promising therapeutic candidates for further clinical evaluation.
Luminex thus enhances the efficiency and precision of translational asthma research and drug discovery.
Discovery and Validation of Non-Clinical Biomarkers
Reliable biomarkers are urgently needed for asthma diagnosis, prognosis, and therapy monitoring. While many studies focus on clinical sample biomarkers, non-clinical research in animal and in vitro models plays a vital role in:
- Discovering candidate biomarkers.
- Validating pathways before clinical translation.
Luminex technology supports:
- Multiplex screening of hundreds of cytokines/chemokines.
- Identification of cytokine patterns correlated with disease severity, treatment response, or pathway activation.
- Generation of robust preclinical datasets to guide clinical biomarker development.
Services you may be interested in:
References:
- Finotto, Susetta. "Resolution of allergic asthma." Seminars in Immunopathology. Vol. 41. No. 6. Berlin/Heidelberg: Springer Berlin Heidelberg, 2019. https://doi.org/10.1007/s00281-019-00770-3
- Debeuf, Nincy, and Bart N. Lambrecht. "Eicosanoid control over antigen presenting cells in asthma." Frontiers in immunology 9 (2018): 2006. https://doi.org/10.3389/fimmu.2018.02006

