In biomedical research, cytokines serve as critical messengers, regulating immunity, inflammation, and cell communication. Accurately measuring cytokines is fundamental to understanding disease mechanisms, discovering biomarkers, and developing new therapies. However, selecting the appropriate cytokine detection method can be challenging. Choosing the wrong approach risks inaccurate data, misinterpretation, and costly project delays.
This guide will walk you through the essential factors and method options, helping you make an informed decision for your specific research needs.
Key Factors to Consider Before Choosing a Cytokine Detection Method
Selecting an appropriate cytokine detection method is not merely a technical decision; it fundamentally shapes the reliability, depth, and interpretation of your results. Understanding your specific project parameters ensures you select a tool that aligns with both scientific and operational demands.
Research Objective
Define your scientific question with precision. Different objectives naturally prioritize different assay features:
- Discovery Research: If your goal is to broadly map cytokine signatures associated with diseases such as cancer, autoimmune disorders, or infectious diseases, you need high-plex platforms capable of detecting tens or hundreds of analytes simultaneously. Here, breadth outweighs absolute quantitation.
- Validation Studies: When confirming the role of select cytokines previously identified, assays that provide high sensitivity, excellent reproducibility, and precise quantitation — even at the expense of multiplexing — are crucial.
- Mechanistic Studies: For projects investigating cellular pathways or cytokine gene regulation, the temporal resolution (how fast changes can be detected) and compatibility with kinetic studies (e.g., time-course experiments) become key selection factors.
Clearly defining your research intent at the outset avoids method mismatches that could compromise your findings later.
Sample Type and Volume
Sample considerations often determine what detection platforms are even viable for a study.
- Sample Type: Different biological samples — serum, plasma, bronchoalveolar lavage fluid (BALF), cerebrospinal fluid (CSF), cell culture supernatants, or tissue lysates — each come with unique challenges. For instance, serum and plasma often contain endogenous cytokine-binding proteins or soluble receptors that can interfere with detection. BALF or tissue homogenates may have lower cytokine concentrations but higher levels of proteases or debris, requiring more sensitive and robust assay systems.
- Sample Volume Constraints: Some research settings, such as pediatric studies or rare disease cohorts, offer only limited volumes of biological material. In these cases, multiplex platforms like Luminex or MSD (Meso Scale Discovery) systems, which require as little as 25–50 μL per test, offer a clear advantage over traditional ELISAs or Western blotting, which typically need 100–200 μL or more.
- Pre-analytical Variables: How samples are collected, processed, and stored (e.g., freeze-thaw cycles, anticoagulant types) can critically affect cytokine stability. Some methods are more tolerant to sample variability than others. For highly sensitive, degradation-prone cytokines, methods with rapid processing capabilities and internal controls for sample integrity are preferred.
Choosing an assay aligned with your available sample type and volume ensures meaningful, reproducible data without overextending precious biological material.
Sensitivity and Dynamic Range
The required detection sensitivity and dynamic range should be carefully matched to the expected cytokine concentrations in your samples.
- Sensitivity Requirements: Cytokine levels vary dramatically depending on the biological context. In acute inflammation, some cytokines like IL-6 or TNF-α can surge to detectable nanogram per milliliter levels, making them accessible to almost any assay. However, regulatory cytokines such as IL-10, or cytokines in healthy baseline samples, often exist at picogram or even sub-picogram concentrations. High-sensitivity assays such as electrochemiluminescence (ECL)-based platforms (e.g., MSD) or ultra-sensitive ELISAs may be essential to accurately measure these low-abundance targets.
- Dynamic Range Considerations: A wide dynamic range minimizes the need for repeated sample dilutions and re-runs. This is especially valuable in longitudinal studies or clinical cohorts where cytokine levels can vary widely across individuals or over time. Platforms like Luminex or MSD often provide 3–5 logs of dynamic range, allowing accurate quantification of both high and low concentration cytokines within the same assay.
- Avoiding the Hook Effect: In extremely high cytokine conditions, like sepsis models, some assays may encounter the "hook effect," where excess cytokine paradoxically reduces the signal. High-dynamic-range platforms and appropriate sample dilutions can mitigate this risk.
Matching the assay's sensitivity and dynamic range to your expected biological conditions ensures both the detection of subtle changes and the avoidance of missed signals or saturation artifacts.
Multiplexing Capability
In studies where multiple cytokines are of interest, multiplexing capability becomes a decisive factor for efficiency, data richness, and cost-effectiveness.
- Benefits of Multiplexing: Immune responses are highly orchestrated events involving networks of cytokines acting synergistically or antagonistically. Measuring multiple cytokines simultaneously from the same sample preserves biological context and captures inter-cytokine relationships that single-analyte assays miss. This systems-level view is particularly valuable in complex diseases like asthma, cancer, or autoimmune disorders.
- Platform Options: Technologies such as Luminex xMAP allow simultaneous detection of 30–500 analytes in a single sample well, dramatically improving throughput and reducing sample consumption. Meso Scale Discovery's U-PLEX system also enables customizable multiplex panels with high sensitivity.
- Limitations to Consider: While multiplex assays offer significant advantages, they are not without trade-offs. Cross-reactivity between detection antibodies, differences in dynamic range between targets, and the potential for signal interference must be carefully evaluated. Validated commercial panels help mitigate these risks, but custom panel development requires rigorous optimization.
- Data Analysis Complexity: Multiplexed datasets generate large, multidimensional outputs. Researchers should be prepared for the added demands of appropriate statistical analysis and bioinformatic interpretation to fully leverage the data.
Selecting a multiplex platform enhances discovery power and conserves resources but demands careful assay validation and thoughtful data analysis to ensure meaningful interpretation.
Specificity and Cross-reactivity
Assay specificity is crucial to ensure accurate cytokine measurement.
- Specificity Concerns: The quality of capture and detection antibodies directly impacts specificity. Poorly optimized antibodies can result in false positives or inaccurate readings. In single-plex assays, specificity is typically high, but in multiplex assays, managing cross-reactivity between cytokines is more challenging.
- Cross-reactivity in Multiplex Assays: Cross-reactivity can occur when an antibody binds to a similar, unintended target. This is a concern in multiplex assays where multiple cytokines are measured simultaneously. Using well-validated, high-quality panels and referring to manufacturer-provided data on cross-reactivity helps minimize this risk.
- Validation and Blocking: Some multiplex assays include blocking agents to reduce non-specific binding. However, it's advisable to confirm findings with orthogonal methods, like single-plex ELISA, to ensure result reliability.
Ensuring high specificity and limiting cross-reactivity is essential for accurate cytokine detection, particularly in multiplex assays.
Throughput and Scalability
The throughput of your cytokine detection method impacts efficiency and costs.
- Low Throughput: For small studies, single-plex ELISA or low-plex assays are cost-effective and easy to implement, suitable for fewer samples and cytokines.
- High Throughput: For large studies, platforms like Luminex and MSD multiplex can process hundreds of samples across many cytokines simultaneously, increasing efficiency.
- Scalability: Choose a platform that can scale with your study's needs, avoiding the need for a method switch as sample size increases.
High-throughput systems enhance efficiency, while scalability ensures that the method can accommodate growing research needs.
Technical Comparison of Major Cytokine Detection Methods
When selecting the appropriate cytokine detection method for your research, understanding the technical capabilities and limitations of each approach is vital. Each method has unique strengths that make it suitable for different applications. Below, we'll compare the most commonly used cytokine detection techniques, focusing on their sensitivity, multiplexing capacity, throughput, dynamic range, sample requirements, and overall performance.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is one of the most widely used techniques for detecting cytokines due to its simplicity, well-established protocols, and high specificity.
- Sensitivity:
ELISA offers good sensitivity for many cytokines, typically in the low picogram per milliliter (pg/mL) range. However, it is generally less sensitive than some newer technologies, such as electrochemiluminescence or Simoa, making it less suitable for detecting low-abundance cytokines like IL-10 or interferon-β. - Multiplexing Capacity:
ELISA is inherently a single-plex method, meaning it can only detect one cytokine per well. However, multiplexing can be achieved by running multiple ELISA tests in parallel, although this significantly increases time, sample consumption, and overall costs. Traditional ELISA is better suited for studies focusing on a single target or a small number of cytokines. - Throughput:
ELISA has moderate throughput. While it is relatively straightforward to implement on 96-well plates, it lacks the scalability needed for high-throughput screening. For studies involving large sample numbers, manual ELISA setups become time-consuming, and the cost per sample can increase. - Dynamic Range and Reproducibility:
ELISA has a moderate dynamic range, typically spanning 2-3 logs, which is suitable for many cytokines but may not be ideal for cytokines with very high or low levels. The reproducibility of ELISA assays is generally high if proper controls are used, though variability can occur due to inconsistencies in reagent quality or handling. - Sample Requirements:
ELISA requires a sample volume of 100–200 μL, which may be restrictive when dealing with limited sample availability, such as in pediatric or animal model studies.
 Schematic representation of four different types of ELISA assays: direct, indirect, sandwich and competitive one (Armengol et al., 2022).
Schematic representation of four different types of ELISA assays: direct, indirect, sandwich and competitive one (Armengol et al., 2022).
Luminex xMAP Technology
Luminex multiplex technology utilizes color-coded microspheres that allow for the detection of multiple cytokines simultaneously. It has become a popular choice for comprehensive cytokine profiling due to its high throughput and flexibility.
- Sensitivity:
Luminex assays provide excellent sensitivity, capable of detecting cytokine concentrations in the low picogram per milliliter (pg/mL) range, with some assays reaching sensitivities in the femtogram per milliliter (fg/mL) range. While not as sensitive as ECL or Simoa platforms for extremely low-abundance targets, it offers sufficient sensitivity for most cytokine studies. - Multiplexing Capacity:
One of Luminex's greatest advantages is its multiplexing capability. It can measure up to 500 analytes in a single sample, depending on the panel. This ability to measure multiple cytokines simultaneously in a single run makes Luminex ideal for studies that require an in-depth look at complex cytokine networks, such as those found in inflammatory diseases or immune profiling. - Throughput:
Luminex supports high-throughput analysis, particularly when combined with automated systems. The ability to process 96- or 384-well plates with minimal hands-on time allows for large-scale studies involving hundreds of samples. This is a major advantage for clinical trials, cohort studies, and biomarker discovery projects. - Dynamic Range and Reproducibility:
Luminex offers a broad dynamic range of 3–5 logs, which allows for the detection of both high and low-concentration cytokines in the same sample. The reproducibility of Luminex assays is generally excellent, provided that careful assay validation and optimization are done. Automated sample handling further minimizes technical variability. - Sample Requirements:
Luminex assays require as little as 25-50 μL of sample, which is advantageous when working with small or precious samples. The small sample volume requirement makes it an ideal choice for pediatric studies or when using animal models with limited sample volume.
Meso Scale Discovery (MSD)
Meso Scale Discovery (MSD) technology is an electrochemiluminescence-based assay that offers high sensitivity and multiplexing, similar to ECL, but with some unique advantages. MSD uses a multi-spot array system that can detect numerous cytokines from a single sample.
- Sensitivity:
MSD platforms provide high sensitivity comparable to that of electrochemiluminescence (ECL), with the ability to detect cytokines at very low levels, including those in the femtogram per milliliter (fg/mL) range. It is particularly effective for detecting cytokines present in complex biological matrices, such as plasma or serum. - Multiplexing Capacity:
MSD systems support high multiplexing, capable of simultaneously measuring 10 to 50 analytes in a single sample. This makes MSD ideal for comprehensive immune profiling studies where researchers need to detect multiple cytokines at once, such as in biomarker discovery or monitoring disease progression. - Throughput:
MSD offers high throughput with customizable plate formats, enabling the processing of hundreds of samples at once. This feature is particularly useful for large-scale studies or clinical trials where the analysis of many samples and analytes is required. Automation tools can further improve efficiency, making MSD suitable for both basic and applied research. - Dynamic Range and Reproducibility:
MSD offers a broad dynamic range (3–5 logs), enabling detection of both low-abundance and high-concentration cytokines in the same assay. The reproducibility of MSD assays is excellent, with minimal inter-assay variation, particularly when optimized protocols are followed. - Sample Requirements:
MSD assays require relatively small sample volumes, typically ranging from 25 to 50 μL, making them suitable for studies with limited sample availability, such as those involving pediatric subjects or animal models.
Flow Cytometry
Flow cytometry is a powerful technique that enables the analysis of cytokine expression at the single-cell level. It is widely used in immunology for profiling immune cell responses, such as those in T cells, B cells, and macrophages.
- Sensitivity:
Flow cytometry offers high sensitivity for detecting cytokines, especially when paired with intracellular staining protocols. It allows the detection of low-abundance cytokines at the single-cell level, making it suitable for studying cytokine production in rare cell populations or in response to specific stimuli. - Multiplexing Capacity:
Flow cytometry allows for multiplexing at the single-cell level. Researchers can measure several cytokines simultaneously within individual cells using a combination of fluorescently tagged antibodies. This approach enables detailed analysis of cytokine production within distinct immune cell subsets and provides insights into complex immune responses. - Throughput:
Flow cytometry supports high throughput, processing thousands of cells per second, and can handle large sample volumes. However, it is generally more suited for studies that focus on cellular cytokine production rather than serum or plasma cytokine levels. Flow cytometry is an excellent choice for immunophenotyping and for analyzing dynamic changes in cytokine production at the cellular level. - Dynamic Range and Reproducibility:
Flow cytometry provides a broad dynamic range, especially when paired with high-sensitivity detectors and appropriate antibody panels. The dynamic range and reproducibility of flow cytometry depend on the instrument and the quality of antibodies used, but it can provide highly accurate data if properly calibrated and optimized. - Sample Requirements:
Flow cytometry requires relatively low sample volumes, especially when working with cell suspensions, making it ideal for experiments with limited sample availability. However, it is less suited for analyzing cytokines in biological fluids like serum, plasma, or cell culture supernatants.
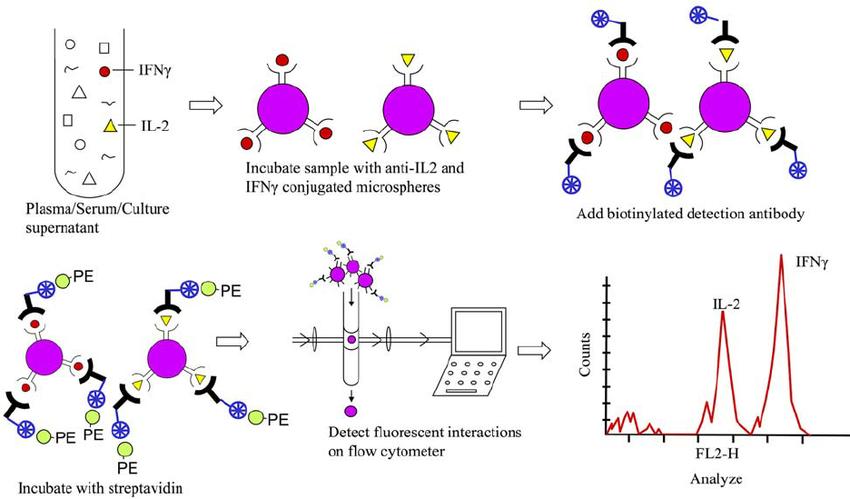 Flow cytometric detection of cytokines in biological specimens (Sachdeva et al., 2007).
Flow cytometric detection of cytokines in biological specimens (Sachdeva et al., 2007).
Single Molecule Array (Simoa)
Simoa technology, based on ultra-sensitive bead-based assays, is designed to achieve ultra-high sensitivity, enabling the detection of cytokines at single-molecule levels.
- Sensitivity:
Simoa is the most sensitive cytokine detection platform currently available, capable of detecting cytokines at attomolar concentrations (as low as single molecules per sample). This makes it ideal for detecting very low-abundance cytokines, particularly in studies of immune regulation or when analyzing rare cytokines that could be indicative of early disease processes. - Multiplexing Capacity:
While Simoa is highly sensitive, its multiplexing capacity is limited compared to Luminex. Simoa can typically measure up to 10-20 cytokines in a single run, though this number is continuously expanding as the technology evolves. While this is adequate for focused studies, it may not be sufficient for broader immune profiling studies involving hundreds of analytes. - Throughput:
Simoa systems are capable of high-throughput analysis but not at the scale of Luminex. The technology is suitable for smaller cohort studies, clinical trials, or research that demands extremely high sensitivity with a moderate number of samples. It is ideal for studies with stringent cytokine detection requirements but may not be cost-effective for large-scale studies involving thousands of samples. - Dynamic Range and Reproducibility:
Simoa provides an extremely broad dynamic range, typically spanning 4-5 logs, similar to Luminex and ECL systems. Its ability to detect single molecules ensures the highest sensitivity and is particularly advantageous for longitudinal studies where subtle cytokine changes need to be detected. - Sample Requirements:
Simoa systems are designed to work with very small sample volumes, often as low as 25 μL, making them ideal for studies involving rare or precious samples. The minimal sample requirement is one of the technology's strongest features, enabling cytokine quantification in challenging sample types.
Matching Your Research Needs to the Right Detection Method
| Feature/Need | ELISA | Luminex xMAP | Meso Scale Discovery (MSD) | Flow Cytometry | Simoa (Single Molecule Array) |
|---|---|---|---|---|---|
| Sensitivity | Moderate (pg/mL range) | High (pg/mL to fg/mL range) | Very High (fg/mL range) | High (Single-cell level) | Ultra-High (fM to aM range) |
| Multiplexing Capability | Single-plex (Can run multiple tests in parallel) | Up to 500 analytes in one run | Up to 50 analytes in one run | Single-cell multiplexing, up to ~10 analytes | Single-plex, multiplexing possible with different assays |
| Throughput | Moderate (96-well plates) | High (96/384-well plates, automated) | High (96/384-well plates, automated) | Very High (Thousands of cells per second) | Moderate (up to 96 samples per run) |
| Dynamic Range | 2-3 logs | 3-5 logs | 3-5 logs | Broad (Cell-level variation) | Extremely wide (5-6 logs) |
| Sample Requirements | Moderate (100-200 μL per sample) | Low (25-50 μL per sample) | Low (25-50 μL per sample) | Low (Cell suspensions) | Extremely low (10-50 μL per sample) |
| Cost | Low to moderate | Moderate to high | High (due to equipment and reagents) | High (equipment and reagents) | High (requires specialized equipment and reagents) |
| Reproducibility | High, with proper controls | Very High (Automated, consistent results) | Very High (if properly optimized) | High (when optimized with correct antibodies) | Very High (due to precise single molecule detection) |
| Suitable For | Small to medium studies, specific cytokine validation | Comprehensive profiling of immune responses | Comprehensive immune profiling with high sensitivity | Single-cell cytokine production analysis, immune cell profiling | Ultra-sensitive analysis of low-abundance cytokines |
| Examples of Cytokines Detected | IL-6, TNF-α, IL-1β, IL-2, IL-4 | IL-6, TNF-α, IFN-γ, IL-10, etc. | IL-6, TNF-α, IL-1β, IFN-γ, IL-10, etc. | IL-2, IFN-γ, IL-4, IL-17 (intracellular, within cells) | IL-1β, TNF-α, IFN-γ, IL-6, and more |
| Best For | Focused cytokine measurement in small samples | High-throughput, multiplexed cytokine profiling in large cohorts | Multiplexed cytokine profiling with ultra-high sensitivity | Immune cell function, intracellular cytokine analysis | Extremely low-abundance cytokines, biomarkers |
| Time Efficiency | Moderate (5-6 hours) | High (can be automated for large sets) | High (automated workflow, quick) | High (real-time analysis, fast processing) | High (rapid, real-time single-molecule detection) |
| Limitations | Single cytokine per well, time-consuming for large panels | Requires optimized protocols for high sensitivity | Expensive, requires significant upfront investment | Requires specialized equipment and expertise, not for serum analysis | Expensive, limited multiplexing, specialized equipment |
| Suitability for Research | Ideal for small studies and validating single cytokines | Ideal for large-scale, complex studies with multiple analytes | Ideal for high sensitivity and multiplexing in complex samples | Ideal for real-time analysis of immune cell behavior | Ideal for research on rare biomarkers and ultra-sensitive detection |
Practical Tips for Reliable Cytokine Detection
- Standardize Sample Handling
Process samples quickly to prevent cytokine degradation. Store at –80°C and limit freeze-thaw cycles. Consistency in collection and storage protocols across samples is essential.
Tip: Add protease inhibitors if necessary and avoid delays between collection and freezing.
- Match Assays to Sample Type
Use assays validated for your specific matrix (e.g., serum, plasma, BALF). Matrix effects can distort results if not properly accounted for.
Tip: Pilot testing can help assess compatibility before full-scale analysis.
- Optimize Standard Curves
Prepare fresh standards for each run and avoid extrapolating beyond the calibration range. Use appropriate curve fitting models to enhance accuracy.
Tip: Always run standards in replicates to monitor consistency.
- Use Rigorous Controls
Include blanks, spiked samples, and internal standards. Controls ensure reliability and help detect drift or technical issues early.
Tip: In multiplex platforms like Luminex or MSD, monitor intra- and inter-assay variability systematically.
- Minimize Manual Error
Automate workflows when possible. If manual pipetting is needed, use calibrated pipettes and consistent technique to reduce variability.
Tip: Process samples in batches to avoid day-to-day fluctuations.
- Contextualize the Data
Cytokine levels can vary with biological factors. Interpret results considering clinical or experimental context, not just absolute values.
Tip: Normalize to appropriate metrics (e.g., protein content) for comparability.
- Confirm Critical Results
Validate key findings with a secondary method, such as confirming ELISA data with flow cytometry or MSD.
Tip: Cross-platform validation strengthens conclusions, especially for low-abundance cytokines.
References:
- Armengol, Eva Sanchez, Aletta Blanka Kerezsi, and Flavia Laffleur. "Allergies caused by textiles: control, research and future perspective in the medical field." International Immunopharmacology 110 (2022): 109043. https://doi.org/10.1016/j.intimp.2022.109043
- Sachdeva, Naresh, and Deshratn Asthana. "Cytokine quantitation: technologies and applications." Front Biosci 12 (2007): 4682-4695.

