Understanding the VEGF Pathway
The Vascular Endothelial Growth Factor (VEGF) pathway is a complex network of signaling molecules that regulates angiogenesis—the formation of new blood vessels. This pathway is crucial for embryonic development, wound healing, and the female reproductive system. At its core, VEGF proteins bind to receptors on the surface of endothelial cells, initiating a cascade of events that lead to the formation of new blood vessels.
VEGF Signaling Pathway Functions
The VEGF signaling pathway is a dynamic and intricately regulated system that plays a pivotal role in various physiological processes. At its core, the primary function of the VEGF pathway is to stimulate angiogenesis, the process by which new blood vessels are formed from existing ones. This function is fundamental for ensuring proper vascular development, tissue growth, and repair.
Angiogenesis and Vascular Development
- Embryonic Development: During embryogenesis, the VEGF signaling pathway is essential for the formation of the vascular system. VEGF proteins, secreted by various cell types, bind to their receptors on endothelial cells, initiating a series of events that result in the sprouting and branching of blood vessels. This orchestrated process is critical for providing developing tissues and organs with an adequate blood supply.
- Postnatal Angiogenesis: In postnatal life, the VEGF pathway continues to regulate angiogenesis, facilitating processes such as wound healing and tissue repair. In response to injuries or ischemia, increased VEGF expression promotes the growth of new blood vessels, ensuring efficient nutrient and oxygen delivery to the affected areas.
Wound Healing and Tissue Repair
- Cell Migration and Proliferation: VEGF signaling is crucial for orchestrating the migration and proliferation of endothelial cells during wound healing. Following tissue injury, VEGF promotes the recruitment of endothelial cells to the site of damage. These cells then proliferate and form new blood vessels, contributing to the restoration of tissue integrity.
- Vascular Permeability: VEGF also enhances vascular permeability, allowing immune cells, nutrients, and growth factors to extravasate from the bloodstream into the surrounding tissue. This increased permeability is a vital aspect of the inflammatory response during wound healing.
Inflammation and Immune Response
- Immune Cell Recruitment: In addition to its role in angiogenesis, the VEGF pathway actively participates in inflammation by promoting the recruitment and activation of immune cells. Increased VEGF levels enhance the migration of immune cells to sites of infection or injury, aiding in the elimination of pathogens and debris.
- Vascular Permeability in Inflammation: During inflammation, VEGF contributes to increased vascular permeability, facilitating the movement of immune cells across the endothelial barrier. This heightened permeability is essential for an efficient immune response by allowing immune cells to reach the affected tissues rapidly.
Regulation of Vascular Homeostasis
- Maintenance of Blood Vessel Integrity: Beyond its role in angiogenesis and inflammation, the VEGF pathway is involved in maintaining vascular homeostasis. VEGF signaling contributes to the regulation of endothelial cell survival, preventing apoptosis and ensuring the integrity of the existing vascular network.
- Neovascularization in Disease: In pathological conditions such as cancer, where uncontrolled angiogenesis is a hallmark, dysregulation of the VEGF pathway contributes to excessive neovascularization. Targeting this pathway with VEGF inhibitors has become a therapeutic strategy in inhibiting tumor growth by disrupting the blood supply.
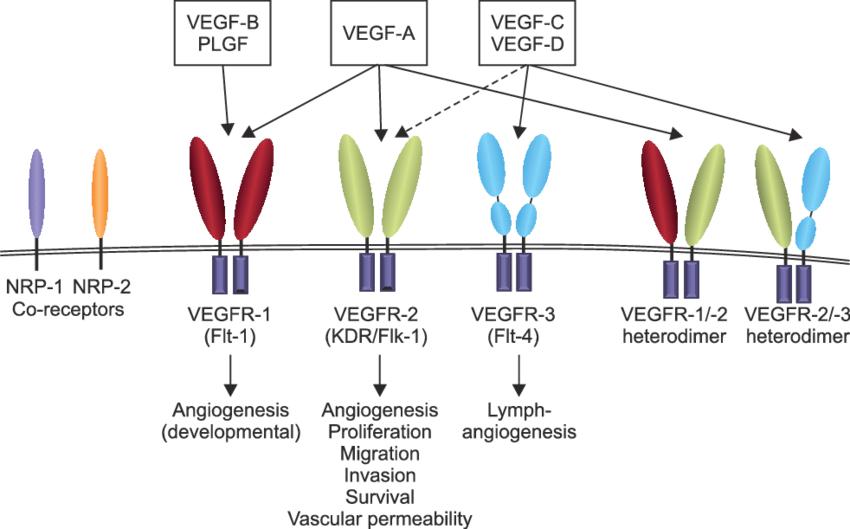 Interactions between VEGF and VEGF receptors, and their biological functions (Bae et al., 2015)
Interactions between VEGF and VEGF receptors, and their biological functions (Bae et al., 2015)
Select Service
Signaling Cascade Mechanism of VEGF
The VEGF signaling pathway is a sophisticated molecular network that orchestrates various cellular responses, primarily focusing on angiogenesis—the formation of new blood vessels. Understanding the intricate signaling cascade of VEGF provides insights into the molecular events governing vascular development, tissue repair, and pathological conditions.
VEGF Receptors and Ligand Binding
- VEGF Receptor Family: The VEGF pathway primarily involves two receptor tyrosine kinases, VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR). These receptors are primarily expressed on the surface of endothelial cells. VEGFR-2 is considered the main mediator of VEGF-induced angiogenesis due to its potent activation by VEGF-A.
- VEGF Ligands: VEGF-A, the most extensively studied isoform, binds to VEGFR-2 with high affinity, initiating the downstream signaling cascade. VEGFR-1, while binding VEGF with lower affinity, also plays a role in modulating the signaling responses.
Receptor Activation and Phosphorylation
- Dimerization and Autophosphorylation: VEGF binding induces dimerization of VEGFR-2, leading to autophosphorylation of specific tyrosine residues in the intracellular kinase domain. This autophosphorylation event is crucial for the activation of the receptor and subsequent initiation of downstream signaling events.
- Activation of Downstream Effectors: The phosphorylated tyrosine residues on VEGFR-2 serve as docking sites for various intracellular signaling molecules, including phospholipase C gamma (PLCγ), PI3K-Akt, and Ras-MAPK. These downstream effectors play pivotal roles in mediating the cellular responses to VEGF.
Ras-MAPK Signaling Pathway
- Activation of Ras: One of the major downstream pathways activated by VEGF is the Ras-MAPK pathway. Binding of VEGF to VEGFR-2 activates the small GTPase Ras, leading to its transition from an inactive GDP-bound state to an active GTP-bound state.
- MAPK Activation: Activated Ras triggers a cascade of events that culminate in the activation of mitogen-activated protein kinases (MAPKs), including ERK1/2. Phosphorylated ERK translocates to the nucleus, where it regulates the expression of genes involved in cell proliferation, survival, and angiogenesis.
PI3K-Akt Signaling Pathway
- Activation of PI3K: VEGFR-2 activation also stimulates Phosphoinositide 3-kinase (PI3K). Activated PI3K converts phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), recruiting Akt to the cell membrane.
- Akt Activation: Akt, a serine/threonine kinase, is then phosphorylated and activated by PIP3. Activated Akt regulates diverse cellular processes, including cell survival, proliferation, and migration, contributing to the pro-angiogenic effects of VEGF.
PLCγ Signaling Pathway
- Activation of PLCγ: VEGF-induced activation of PLCγ is another critical signaling pathway. PLCγ hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG).
- Intracellular Calcium Release: IP3 triggers the release of intracellular calcium, leading to the activation of protein kinase C (PKC). These events contribute to the regulation of endothelial cell permeability and migration.
Integration of Signaling Pathways
- Cross-talk between Pathways: The Ras-MAPK, PI3K-Akt, and PLCγ pathways are interconnected and often exhibit cross-talk. This intricate integration allows VEGF to orchestrate a synergistic cellular response, including cell proliferation, survival, migration, and angiogenesis.
Transcriptional Regulation
- Nuclear Events: The activation of downstream effectors leads to the transcriptional regulation of genes involved in angiogenesis and endothelial cell function. This includes the expression of vascular endothelial cadherin (VE-cadherin), endothelial nitric oxide synthase (eNOS), and various pro-angiogenic factors.
Mechanism of Action of VEGF Inhibitors
VEGF inhibitors like bevacizumab and aflibercept selectively disrupt the VEGF signaling pathway by targeting VEGF ligands (VEGF-A, etc.) and their receptors, primarily VEGFR-1 and VEGFR-2, on endothelial cells.
Binding Inhibition: VEGF inhibitors directly bind to VEGF ligands, obstructing their interaction with receptors and curtailing VEGFR activation. This disruption effectively hinders downstream signaling cascades.
Downregulation of Angiogenesis: Inhibitors suppress angiogenesis, crucial in pathological conditions like cancer, by effectively reducing endothelial cell proliferation and migration, limiting the formation of new blood vessels.
Disruption of Tumor Microenvironment: By limiting angiogenesis, inhibitors disrupt the tumor microenvironment, hindering nutrient accessibility and impairing the delivery of chemotherapeutic agents to the tumor site.
Normalization of Tumor Vasculature: VEGF inhibitors contribute to normalized tumor vasculature, improving the efficiency of drug delivery and oxygenation within tumors.
Management of Vascular Permeability: In addition to their anti-angiogenic effects, VEGF inhibitors manage vascular permeability, mitigating edema and enhancing overall vascular integrity.
Luminex Multiplex Technology for VEGF Signaling Pathway Analysis
The VEGF signaling pathway, being multifaceted, involves a network of signaling molecules and mediators. Luminex technology allows for the simultaneous quantification of key components such as VEGF-A, VEGFR-1, VEGFR-2, and downstream effectors like phosphorylated ERK and Akt. This comprehensive profiling enhances our ability to decipher the intricate interplay of molecules within the pathway.
Luminex assays provide not only qualitative but also quantitative data, allowing researchers to discern the relative abundance of different signaling molecules in response to various stimuli. This quantitative precision is crucial for understanding the dynamics of VEGF signaling under different experimental conditions and disease states.
Beyond the core components of the VEGF pathway, Luminex technology enables the identification of additional biomarkers associated with pathway activation. This multiplexed approach allows for the discovery of novel regulatory factors, providing valuable insights into potential therapeutic targets or diagnostic markers associated with VEGF-related pathologies.
The sensitivity and specificity of Luminex technology contribute to its effectiveness in detecting low-abundance molecules within the VEGF pathway. This capability is particularly relevant when studying subtle variations in signaling events or when working with limited sample volumes, such as in clinical studies or rare cell populations.
Traditional methods of analyzing the VEGF signaling pathway often involve separate assays for individual components, demanding significant time and resources. Luminex technology streamlines this process by consolidating multiple assays into a single platform, reducing the time and reagents required for comprehensive pathway analysis.
Select Service
Reference:
- Bae, Ok-Nam, et al. "Keratinocytic vascular endothelial growth factor as a novel biomarker for pathological skin condition." Biomolecules & therapeutics 23.1 (2015): 12.