Microbial infections are a significant public health concern worldwide, and detecting and identifying infectious agents are essential for diagnosis and treatment. Traditional microbiological assays are time-consuming, laborious, and lack sensitivity and specificity. As a result, there is a growing need for high-throughput, multiplexed technologies for detecting and identifying microorganisms in clinical and research settings. Luminex technology, a bead-based suspension array platform, is one such technology that has gained popularity in recent years. It revolutionized the field of microbiology by providing a fast and sensitive way to detect multiple pathogens simultaneously.
The Principle of Luminex Technology
Luminex technology uses two types of bead-based multiplexing assays: Luminex xMAP and Luminex xTAG.
In the Luminex xMAP technology, beads of different colors are coated with different capture molecules that bind to different analytes. The beads are then incubated with a sample containing the target analytes, and the beads capture the analytes of interest. A biotinylated detection antibody is then added to the beads, which binds to the captured analytes. Finally, a fluorescently labeled streptavidin is added, which binds to the biotinylated antibody, generating a fluorescent signal that is detected by a flow cytometer. The signal intensity of each bead is proportional to the amount of the analyte captured, allowing the detection and quantification of multiple analytes simultaneously.
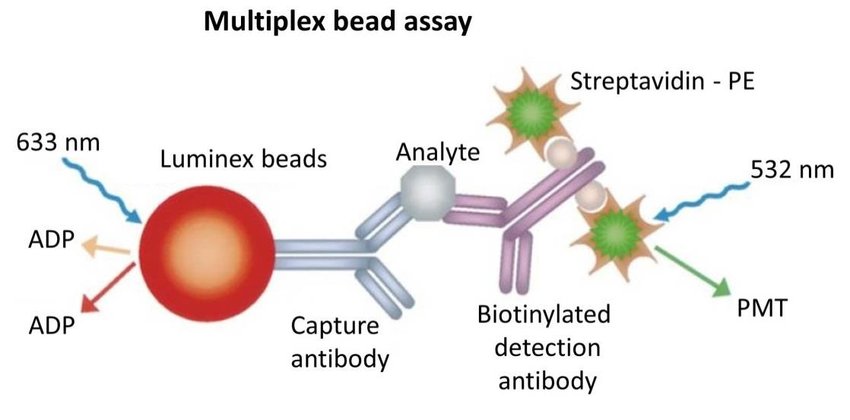 Bead-based multiplex assay using Luminex technology (Kuen et al., 2017)
Bead-based multiplex assay using Luminex technology (Kuen et al., 2017)
In Luminex xTAG technology, oligonucleotide-tagged beads are used instead of colored beads. The capture molecules are coupled to the beads, and the sample is incubated with the beads. The captured analytes are then detected using a fluorescently labeled oligonucleotide probe, which hybridizes to the tag on the captured bead, generating a fluorescent signal. The signal intensity of each bead is proportional to the amount of the analyte captured, allowing the detection and quantification of multiple analytes simultaneously.
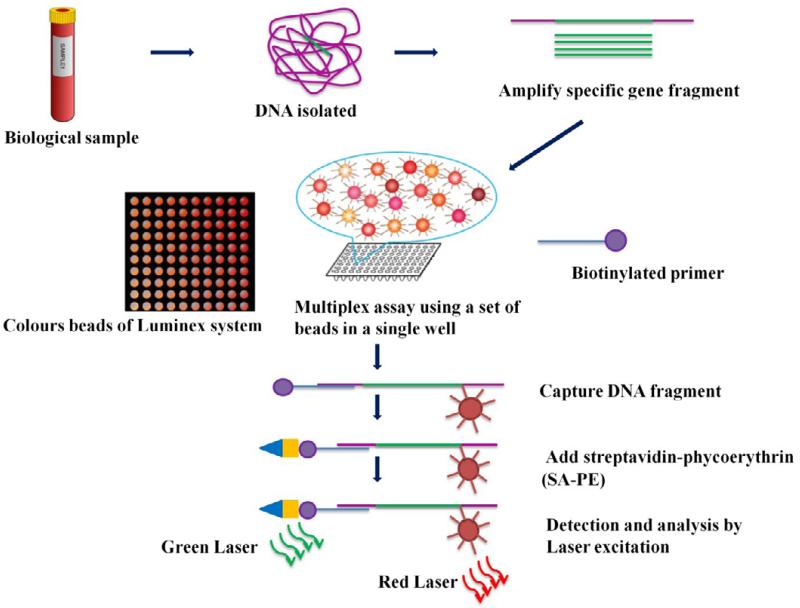 Principle of Luminex assay (Ranjan et al., 2015)
Principle of Luminex assay (Ranjan et al., 2015)
Luminex technology has several advantages over traditional microbiological assays. It allows the detection of multiple analytes in a single sample, reducing the time and cost of testing. The technology is highly sensitive and specific, allowing the detection of low concentrations of analytes. Additionally, Luminex technology requires less sample volume than traditional assays, making it an attractive option for samples that are limited or difficult to obtain.
Application of Luminex Technology in Multiplex Microbiological Assays
Luminex technology has been widely used in microbiological assays, including the detection and identification of bacterial and viral pathogens, as well as the assessment of immune responses to infectious agents. The technology has been used to detect and identify bacterial and viral pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae, Mycobacterium tuberculosis, and influenza viruses. In addition, Luminex technology has been used to assess the immune response to infectious agents, such as measuring cytokine and chemokine levels in response to infection.
Luminex technology has also been used in the diagnosis and monitoring of infectious diseases. For example, in HIV-infected patients, Luminex technology has been used to measure viral load and drug resistance mutations, allowing for personalized treatment plans. In addition, Luminex technology has been used to detect and monitor the progression of infections, such as cytomegalovirus infections in transplant recipients.
For instance, in the study by Fawcett et al. (2006), Luminex technology was used for rapid and high-throughput multiplexed nucleic acid detection of respiratory viruses. The assay detected nine respiratory viruses in a single reaction and had a sensitivity of 90.6% and a specificity of 100%.
Similarly, Luminex technology has been applied in the detection of Iris yellow spot virus (IYSV), a virus that affects onion crops. In the study by Liu et al. (2018), a Luminex xMAP-based microsphere immunoassay was developed for specific detection of IYSV. The assay had a detection limit of 100 fg of purified IYSV, making it highly sensitive and specific for IYSV detection.
Advantages of Luminex Technology
Luminex technology offers several advantages over traditional microbiological assays.
- The technology enables the detection of multiple pathogens simultaneously, reducing the time and cost associated with traditional methods.
- Luminex technology is highly sensitive, allowing for the detection of even low-level infections.
- The technology is highly specific, enabling the differentiation of closely related pathogens.
- The technology is compatible with various sample types, including blood, serum, plasma, urine, and feces, among others.
- Luminex technology is fully automated, reducing the likelihood of operator error and ensuring consistent results.
Luminex technology is a powerful tool in the field of microbiology, enabling the rapid and sensitive detection of multiple pathogens simultaneously. The technology has been applied in various microbiological assays, including nucleic acid detection, immunoassays, and serological assays, among others.
Creative Proteomics is a leading provider of Luminex technology-based services. The company's team of experienced scientists utilizes the latest Luminex technology to provide high-quality and reliable results, helping clients to identify and manage microbial infections effectively. Additionally, Creative Proteomics offers customized solutions tailored to meet specific client needs, making it an ideal partner for researchers and clinicians in the field of microbiology.
References:
- Kuen, Janina. Influence of 3D tumor cell/fibroblast co-culture on monocyte differentiation and tumor progression in pancreatic cancer. Diss. Universität Würzburg, 2017.
- Ranjan, Koushlesh, P. Minakshi, and Gaya Prasad. "Application of molecular and serological diagnostics in veterinary parasitology." J. Adv. Parasitol 2.4 (2015): 80-99.