The recognition of viral infections is a critical aspect of the immune response, and one of the key players in this process is the RLR (RIG-I-like receptor) signaling pathway. RLRs are a family of cytoplasmic pattern recognition receptors that play a pivotal role in detecting viral RNA and initiating immune responses.
The RLR signaling pathway includes prominent members such as RIG-I (Retinoic Acid-Inducible Gene I) and MDA5 (Melanoma Differentiation-Associated protein 5). These receptors are known for their ability to recognize distinct viral RNA patterns, serving as sentinels against a wide array of viral invaders.
Understanding the intricacies of RLR signaling is crucial for unraveling the mechanisms by which our immune system responds to viral threats. This study aims to delve into the molecular details of RLR activation, the downstream signaling events, and the resulting cytokine production.
The significance of this research lies in its potential to uncover novel therapeutic targets for antiviral interventions. By elucidating the RLR signaling pathway, we can gain insights into how to modulate immune responses effectively, contributing to the development of innovative strategies to combat viral infections. Moreover, a comprehensive understanding of RLRs adds to our broader knowledge of innate immunity, paving the way for advancements in immunology research and potential applications in clinical settings.
What are RIG-I-like receptors?
RIG-I-like receptors (RLRs) constitute a vital component of the innate immune system, specifically recognized for their role in sensing and responding to viral infections. RLRs are a family of cytoplasmic pattern recognition receptors that play a crucial part in the early detection of viral RNA. The primary members of the RLR family include RIG-I (Retinoic Acid-Inducible Gene I) and MDA5 (Melanoma Differentiation-Associated protein 5).
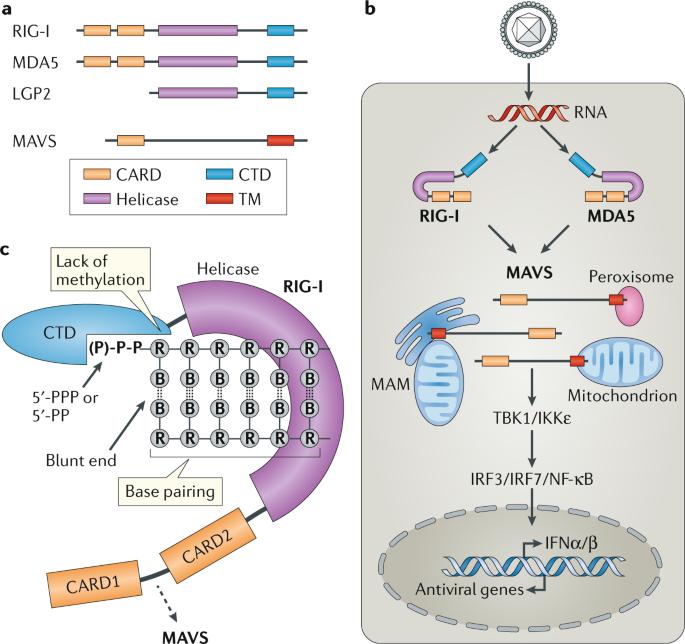 RIG-I-like receptors (Rehwinkel et al., 2020)
RIG-I-like receptors (Rehwinkel et al., 2020)
What Do RLRs Mainly Detect?
RIG-I-like receptors (RLRs) primarily detect viral RNA structures within the cytoplasm of host cells. These receptors specifically recognize distinct patterns associated with viral nucleic acids, serving as vigilant guardians against viral infections. The key molecular features that RLRs target include:
1. 5'-triphosphate RNA: RIG-I is particularly attuned to short double-stranded RNA molecules bearing a 5'-triphosphate moiety. This structure is a common hallmark of many RNA viruses, and its recognition by RIG-I initiates the signaling cascade leading to antiviral responses.
2. Long Double-Stranded RNA: MDA5 specializes in detecting long double-stranded RNA, a feature shared by certain RNA viruses. The recognition of this specific RNA structure by MDA5 contributes to the activation of immune responses against a broader range of viral pathogens.
Select Service
Association of RLRs with Pathogen Recognition
The association between RLRs and pathogen recognition is integral to the early defense mechanisms of the immune system. RLRs play a pivotal role in recognizing and distinguishing viral RNA from host RNA, thereby initiating a rapid and targeted response to viral infections.
Upon detection of viral RNA, RLRs trigger signaling pathways that lead to the activation of transcription factors and the production of antiviral cytokines. This process not only establishes an antiviral state within infected cells but also communicates the presence of a viral threat to neighboring cells, contributing to the overall coordination of the immune response.
The ability of RLRs to discern specific viral RNA structures underscores their importance as pattern recognition receptors in the immune system. Understanding the intricacies of RLR-pathogen interactions provides valuable insights into the host's ability to discriminate between self and non-self, contributing to our broader comprehension of innate immune responses against viral pathogens.
Role of RLRs in the Immune System
RIG-I-like receptors (RLRs) occupy a central position in the intricate network of the immune system, orchestrating a rapid and targeted response against viral infections. Their roles encompass:
- Initiation of Antiviral Signaling: RLR activation leads to signaling pathways that induce type I interferons and proinflammatory cytokines, establishing an antiviral state.
- Interferon-Mediated Antiviral State: Type I interferons restrict viral replication, contributing to innate immune defense.
- Link to Adaptive Immunity: RLR activation enhances the overall immune response by contributing to dendritic cell activation and the presentation of viral antigens to T cells.
Cell Types Expressing RLRs
RLRs are expressed in a cell type-specific manner, with their presence observed in various cell types crucial for immune surveillance. These include:
- Epithelial Cells: RLRs are expressed in epithelial cells lining mucosal surfaces, providing a frontline defense against viral invasion at mucosal entry points.
- Fibroblasts: Fibroblasts, essential components of connective tissues, express RLRs, contributing to the immune surveillance in the extracellular matrix.
- Immune Cells: Certain immune cells, such as macrophages and dendritic cells, express RLRs, enabling them to actively participate in the detection and response to viral infections.
- Endothelial Cells: Cells lining blood vessels, known as endothelial cells, also express RLRs, playing a role in the immune surveillance within the circulatory system.
Distinction Between RLRs and Other Immune Receptors
Comparison of TLRs (Toll-like Receptors) and RLRs
Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs) are two distinct families of pattern recognition receptors that play pivotal roles in the immune system. Here, we highlight key differences between TLRs and RLRs:
1. Localization:
- TLRs: TLRs are predominantly expressed on the cell surface and within endosomes, where they recognize a wide range of pathogen-associated molecular patterns (PAMPs), including bacterial cell wall components and viral nucleic acids.
- RLRs: RLRs are localized within the cytoplasm of host cells, specifically recognizing viral RNA structures, such as 5'-triphosphate and long double-stranded RNA.
2. RNA Recognition:
- TLRs: TLRs primarily recognize a variety of microbial components, including lipopolysaccharides, lipoproteins, and nucleic acids from bacteria and viruses.
- RLRs: RLRs specifically detect viral RNA structures, making them key players in the early detection of viral infections.
3. Downstream Signaling Pathways:
- TLRs: TLR activation leads to the activation of signaling pathways involving adaptor molecules like MyD88 and TRIF, ultimately resulting in the production of proinflammatory cytokines and type I interferons.
- RLRs: RLRs initiate signaling cascades involving MAVS (mitochondrial antiviral-signaling protein), culminating in the activation of transcription factors and the production of antiviral cytokines.
Differential Roles in Immune Responses
The distinct properties of TLRs and RLRs contribute to their differential roles in immune responses:
1. Pathogen Specificity:
- TLRs: TLRs exhibit broad specificity, recognizing a diverse array of microbial components, contributing to the defense against a wide range of pathogens.
- RLRs: RLRs, with their specific recognition of viral RNA, specialize in detecting and responding to viral infections.
2. Cellular Localization:
- TLRs: TLRs are positioned on the cell surface and endosomes, enabling the detection of extracellular and endocytosed pathogens.
- RLRs: RLRs are located within the cytoplasm, allowing them to survey the internal environment for viral intrusion.
3. Adaptive Immune Connection:
- TLRs: TLRs play a role in shaping adaptive immune responses by promoting the maturation of antigen-presenting cells and influencing T cell activation.
- RLRs: RLRs also contribute to adaptive immunity by facilitating the presentation of viral antigens to T cells through the activation of dendritic cells.
Expression Locations of RLRs
Intracellular Localization of RLRs
RIG-I-like receptors (RLRs) are strategically positioned within the cytoplasm of host cells, ensuring their proximity to potential viral RNA intruders. This intracellular localization is essential for the detection of viral RNA during various stages of the viral life cycle. Key aspects of the intracellular localization include:
- Cytoplasmic Surveillance: RLRs, such as RIG-I and MDA5, actively survey the cytoplasmic environment for the presence of viral RNA. This positioning allows them to promptly respond to viral infections by initiating signaling cascades upon RNA recognition.
- Association with Membrane Structures: RLRs are known to associate with cellular membrane structures, such as mitochondria and peroxisomes. This association facilitates interactions with signaling adaptor proteins, such as MAVS (mitochondrial antiviral-signaling protein), triggering downstream signaling events.
Differential Expression of RLRs in Various Cell Types
The expression of RLRs varies across different cell types, reflecting the diverse roles these receptors play in immune surveillance. Understanding these expression differences provides insights into the adaptability of the immune response. Key considerations include:
- Epithelial Cells: Epithelial cells lining mucosal surfaces express RLRs, providing a frontline defense against viral invasion at mucosal entry points.
- Fibroblasts: Fibroblasts, essential components of connective tissues, express RLRs, contributing to immune surveillance in the extracellular matrix.
- Immune Cells: Certain immune cells, including macrophages and dendritic cells, express RLRs, allowing them to actively participate in the detection and response to viral infections.
- Endothelial Cells: Cells lining blood vessels, known as endothelial cells, also express RLRs, playing a role in immune surveillance within the circulatory system.
Cytokines in the RLRs Signaling Pathway
Key Cytokines in the RLRs Signaling Pathway
The RIG-I-like receptor (RLR) signaling pathway is intricately linked to the production and regulation of key cytokines, which are instrumental in orchestrating the immune response against viral infections. The critical cytokines involved in the RLRs signaling pathway include:
- Type I Interferons (IFNs): RLR activation leads to the robust induction of type I interferons, such as interferon-alpha (IFN-α) and interferon-beta (IFN-β). These cytokines are pivotal in establishing an antiviral state within infected and neighboring cells, hindering viral replication.
- Proinflammatory Cytokines: RLR signaling stimulates the production of various proinflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β). These cytokines contribute to the inflammatory milieu, recruiting immune cells and enhancing antiviral defenses.
- Chemokines: RLR activation induces the release of chemokines, such as C-X-C motif chemokine ligands (CXCLs) and C-C motif chemokine ligands (CCLs). These chemokines play a crucial role in recruiting immune cells to the site of infection, aiding in the clearance of the virus.
Regulation of Cytokines in the Immune Response
Cytokines generated through the RLRs signaling pathway play diverse roles in regulating immune responses:
- Antiviral Defense: Type I interferons generated in response to RLR activation establish an antiviral state in cells, inhibiting viral replication and spread.
- Immune Cell Activation: Proinflammatory cytokines contribute to the activation of immune cells, enhancing their ability to recognize and eliminate virus-infected cells.
- Inflammatory Response Modulation: Cytokines regulate the intensity and duration of the inflammatory response, ensuring a controlled and effective immune reaction without excessive tissue damage.
- Chemotaxis and Immune Cell Recruitment: Chemokines facilitate the recruitment of immune cells to the site of infection, promoting a coordinated and targeted immune response.
Select Service
Reference:
- Rehwinkel, Jan, and Michaela U. Gack. "RIG-I-like receptors: their regulation and roles in RNA sensing." Nature Reviews Immunology 20.9 (2020): 537-551.