What are Porcine Cytokines?
Cytokines are a broad class of small proteins that are essential to the immune system's communication network, mediating and regulating immune responses, inflammation, and cell development. Found in all mammals, cytokines act as messengers that convey signals between cells, coordinating the body's defense mechanisms against infections, injuries, and other stressors.
Pigs, like other mammals, rely on a complex and finely tuned immune system to combat the array of pathogens they encounter, especially in intensive farming environments where disease pressure is high. Cytokines are central to this system, influencing how pigs respond to infections, manage inflammation, and maintain overall health. They are produced by various cell types, including immune cells such as macrophages, T cells, and B cells, as well as non-immune cells like endothelial and epithelial cells.
The pig immune system shares significant similarities with the human immune system, making pigs an excellent model for studying human diseases and immune responses. This similarity is leveraged in biomedical research, particularly in areas like xenotransplantation and the development of vaccines and therapeutics.
Cytokines influence key processes such as growth, reproduction, and response to vaccines. A deep understanding of these molecules can lead to better strategies for disease prevention, enhanced vaccine efficacy, and improved animal welfare, all of which are essential for the sustainability and productivity of pig farming.
Services you may be interested in:
Classification and Types of Porcine Cytokines
Pro-inflammatory Cytokines
Pro-inflammatory cytokines are essential in initiating and sustaining the inflammatory response. They are typically produced in response to infection or tissue injury and help to recruit immune cells to the site of damage. In pigs, key pro-inflammatory cytokines include:
- Interleukin-1 (IL-1): IL-1α and IL-1β are critical mediators of the inflammatory response, inducing fever and the expression of other pro-inflammatory molecules.
- Interleukin-6 (IL-6): IL-6 is involved in stimulating acute-phase protein production and supporting the proliferation of B cells.
- Tumor Necrosis Factor-α (TNF-α): TNF-α is a potent pro-inflammatory cytokine that can induce apoptosis, fever, and inflammation.
These cytokines are particularly important during infections with pathogens such as Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), where they help control viral replication but can also contribute to tissue damage and disease severity.
Anti-inflammatory Cytokines
Anti-inflammatory cytokines are crucial for limiting and resolving inflammation, preventing excessive tissue damage. In pigs, prominent anti-inflammatory cytokines include:
- Interleukin-10 (IL-10): IL-10 is a key regulator of immune responses, inhibiting the production of pro-inflammatory cytokines and promoting the survival of regulatory T cells.
- Transforming Growth Factor-β (TGF-β): TGF-β is involved in regulating cell growth, differentiation, and apoptosis. It also plays a significant role in maintaining immune homeostasis by controlling the activation and proliferation of immune cells.
These cytokines are vital for preventing chronic inflammation and autoimmune responses, ensuring that the immune response does not overshoot and cause harm to the host.
Interferons in Pigs
Interferons (IFNs) are a group of cytokines that are primarily involved in antiviral defense. They are classified into three types: Type I (e.g., IFN-α, IFN-β), Type II (IFN-γ), and Type III (IFN-λ). In pigs:
- IFN-α and IFN-β: These are produced in response to viral infections and play a crucial role in the early stages of antiviral immunity by inducing the expression of antiviral proteins and enhancing the antigen presentation process.
- IFN-γ: This cytokine is produced by T cells and natural killer (NK) cells, and it is essential for activating macrophages and enhancing the cytotoxic activity of NK cells.
Interferons are particularly important in the defense against viral infections like swine influenza and African swine fever virus (ASFV).
Growth Factors
Growth factors are a subset of cytokines that primarily regulate cell growth, proliferation, and differentiation. In pigs:
- TGF-β: Beyond its role in immune regulation, TGF-β is also crucial in tissue repair and fibrosis, influencing the healing process after injury or infection.
Understanding the role of growth factors in pigs is essential for enhancing wound healing and tissue regeneration, which can be crucial in both agricultural and clinical settings.
Chemokines
Chemokines are a specific class of cytokines responsible for directing the movement of immune cells to sites of infection or injury. In pigs, chemokines such as:
- CXCL8 (IL-8): Attracts neutrophils to sites of infection.
- CCL2 (MCP-1): Recruits monocytes and T cells to inflamed tissues.
Chemokines are critical for coordinating the immune response during infection, ensuring that the appropriate immune cells are present where they are needed most.
Porcine Cytokine Gene Expression
The regulation of cytokine gene expression in pigs is a complex and tightly controlled process, involving multiple layers of control that ensure cytokines are produced at the right time, in the right amount, and in response to the appropriate stimuli. This regulation occurs at several levels, including transcriptional control, mRNA stability, and post-translational modifications, each contributing to the precise modulation of cytokine production.
At the transcriptional level, cytokine gene expression is primarily controlled by transcription factors, which are proteins that bind to specific DNA sequences near the cytokine genes and either promote or inhibit their transcription into messenger RNA (mRNA). Among the most critical transcription factors involved in cytokine regulation are NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells), AP-1 (Activator Protein 1), and STATs (Signal Transducer and Activator of Transcription proteins). These transcription factors are activated by various signaling pathways triggered by immune challenges, such as infections or tissue injury. For example, in response to a pathogen, NF-κB is activated and translocates to the nucleus, where it binds to the promoters of pro-inflammatory cytokine genes like TNF-α and IL-1β, initiating their transcription.
In addition to transcriptional control, cytokine gene expression is influenced by epigenetic modifications. Epigenetics involves changes in gene expression that do not alter the underlying DNA sequence but affect how the DNA is packaged within the cell. Key epigenetic mechanisms include DNA methylation and histone modifications. DNA methylation typically suppresses gene expression by adding methyl groups to the DNA, which can prevent the binding of transcription factors to the gene's promoter. Histone modifications, such as acetylation and methylation, alter the structure of chromatin—the complex of DNA and proteins within the nucleus—making it either more or less accessible for transcription. In the context of cytokine genes, these epigenetic changes can either enhance or repress the production of cytokines, depending on the specific needs of the immune response.
Another critical layer of regulation is the control of mRNA stability. After a cytokine gene is transcribed, the stability and lifespan of its mRNA can significantly influence how much of the cytokine protein is produced. Certain cytokine mRNAs contain sequences, often in their 3' untranslated regions (UTRs), that determine how quickly they are degraded. Proteins that bind to these regions can either stabilize the mRNA, allowing for more prolonged and increased cytokine production, or destabilize it, leading to rapid degradation and reduced cytokine levels. This mechanism allows cells to fine-tune cytokine production dynamically in response to changing conditions.
Post-translational modifications also play a role in regulating cytokine activity. Once cytokines are synthesized, they often undergo modifications such as glycosylation, phosphorylation, or proteolytic cleavage, which can influence their stability, activity, and interactions with receptors. For example, glycosylation can protect cytokines from degradation in the extracellular environment, prolonging their activity and enhancing their ability to signal other cells.
How to Detect Porcine Cytokine Levels?
Detecting cytokine levels in pigs is crucial for studying immune responses, disease mechanisms, and evaluating vaccine efficacy. Various techniques can be used to measure cytokine levels, including enzyme-linked immunosorbent assays (ELISA), quantitative real-time PCR (qPCR), flow cytometry, and the increasingly popular Luminex xMAP technology. Each method has its advantages and applications, but Luminex xMAP technology is particularly valued for its high-throughput and multiplexing capabilities.
Luminex xMAP Technology
Luminex xMAP technology is a bead-based multiplexing system that enables simultaneous detection of multiple cytokines. The technology uses color-coded microspheres, each coated with antibodies specific to different cytokines. When a sample is added, cytokines in the sample bind to their corresponding antibodies on the microspheres. Afterward, a detection antibody labeled with a fluorescent tag is added, forming a "sandwich" complex with the bound cytokines.
The Luminex instrument then uses lasers to analyze the microspheres. It identifies the cytokines based on the color-coded microspheres and quantifies the cytokine levels based on the fluorescence intensity. This method allows for the simultaneous measurement of up to 50 or more cytokines in a single assay, greatly enhancing experimental efficiency and data consistency.
Luminex xMAP technology is particularly useful for studying complex immune responses, such as assessing the breadth and intensity of immune responses in vaccine development or monitoring cytokine profiles in disease research. Its high sensitivity and specificity make it ideal for experiments requiring the measurement of multiple low-concentration cytokines simultaneously. Additionally, Luminex xMAP requires smaller sample volumes compared to traditional ELISA, which is advantageous for working with limited or precious samples, such as those from neonatal or diseased pigs.
Services you may be interested in:
Comparison with Other Methods
While Luminex xMAP offers significant advantages, other methods like ELISA remain the gold standard for measuring single cytokines. ELISA is straightforward and well-suited for determining the absolute concentration of specific cytokines. However, for studies requiring a comprehensive view of multiple cytokines or their interactions, ELISA's limitations become apparent. qPCR is useful for assessing cytokine gene expression levels, providing indirect information about cytokine production, while flow cytometry can simultaneously analyze cytokine levels and cell surface markers.
The choice of detection method depends on research objectives, sample types, and the required resolution and throughput. Luminex xMAP technology stands out for its multiplexing capabilities and efficiency, making it an indispensable tool in modern immunology research, particularly for measuring cytokine levels in pigs. Its ability to provide comprehensive cytokine profiles in a single assay enhances our understanding of immune responses and disease processes, supporting advances in veterinary medicine and swine health management.
Role of Cytokines in Porcine Diseases
Viral Infections
Viral infections in pigs often trigger robust cytokine responses that can both control the infection and contribute to disease severity. For instance, in Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) infection, cytokines such as TNF-α, IL-1β, and IL-6 are significantly elevated. These pro-inflammatory cytokines are critical in initiating the immune response, helping to control viral replication by activating immune cells like macrophages and promoting the clearance of infected cells. However, their overproduction can lead to extensive inflammation, particularly in the lungs, exacerbating respiratory symptoms and contributing to the severe respiratory distress associated with PRRSV.
Similarly, during Swine Influenza Virus (SIV) infections, the cytokine response is characterized by a rapid increase in IFN-α, IFN-β, and other pro-inflammatory cytokines. These cytokines are essential for antiviral defense, particularly in the early stages of infection, as they enhance the killing of infected cells and inhibit viral replication. However, the resulting inflammation can also damage lung tissues, leading to symptoms such as coughing, fever, and difficulty breathing. In severe cases, the cytokine-induced inflammation can progress to acute respiratory distress syndrome (ARDS), a life-threatening condition.
In African Swine Fever Virus (ASFV) infection, the cytokine response is even more complex. ASFV induces a mixed cytokine profile, with both pro-inflammatory cytokines like TNF-α and IL-1β and anti-inflammatory cytokines like IL-10 being upregulated. The pro-inflammatory cytokines are involved in controlling the virus, but they also contribute to the severe hemorrhagic fever and widespread tissue damage characteristic of ASFV. The concurrent upregulation of anti-inflammatory cytokines, while potentially an attempt by the immune system to limit damage, can lead to immune suppression, making it difficult for the host to clear the virus and contributing to the high mortality associated with ASFV.
Bacterial Infections
Cytokines are equally important in the immune response to bacterial infections in pigs. For example, in infections caused by Mycoplasma hyopneumoniae, the causative agent of porcine enzootic pneumonia, cytokines such as IL-1β, IL-6, and TNF-α are produced in response to bacterial invasion. These cytokines recruit immune cells like neutrophils and macrophages to the site of infection, which is critical for controlling the bacteria. However, this recruitment also leads to the accumulation of immune cells in the lungs, contributing to the characteristic lung lesions and chronic inflammation associated with the disease. The prolonged cytokine production and resulting inflammation can impair lung function and reduce growth rates in affected pigs.
In the case of Streptococcus suis infection, cytokine dysregulation is a significant factor in the disease's progression. S. suis can cause a range of conditions, including meningitis, septicemia, and arthritis. During infection, the bacteria trigger the release of cytokines such as IL-6, TNF-α, and IL-10. While IL-6 and TNF-α are necessary for controlling the infection, their overproduction can lead to excessive inflammation, contributing to the severe symptoms of meningitis, such as brain inflammation and tissue damage. Additionally, IL-10, an anti-inflammatory cytokine, is often upregulated in an attempt to counterbalance the inflammation, but its effects can also suppress the immune response, potentially allowing the bacteria to persist and cause chronic infection.
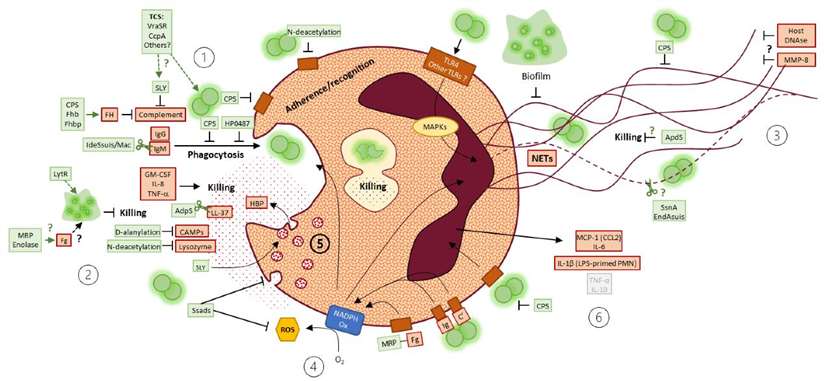 Mechanisms of S. suis interference with neutrophil functions (including findings from porcine, murine and human models) (Bleuzé et al., 2021).
Mechanisms of S. suis interference with neutrophil functions (including findings from porcine, murine and human models) (Bleuzé et al., 2021).
Autoimmune and Inflammatory Diseases
Cytokine dysregulation is also a hallmark of autoimmune and inflammatory diseases in pigs. In conditions such as Glässer's Disease, caused by Haemophilus parasuis, excessive cytokine production leads to polyserositis, arthritis, and meningitis. The disease is characterized by the overproduction of pro-inflammatory cytokines like TNF-α and IL-6, which drive the inflammatory process. This excessive inflammation results in the accumulation of fluid in body cavities (polyserositis), severe joint inflammation (arthritis), and inflammation of the meninges (meningitis), leading to significant morbidity and, in severe cases, death.
Porcine Dermatitis and Nephropathy Syndrome (PDNS) is another example where cytokines play a critical role in disease development. PDNS is believed to be immune-mediated and is associated with an imbalance between pro-inflammatory and anti-inflammatory cytokines. Elevated levels of pro-inflammatory cytokines, particularly TNF-α and IL-6, contribute to the systemic inflammation observed in PDNS, leading to skin lesions and kidney damage. The chronic inflammation associated with this cytokine imbalance can result in significant tissue damage and is a major cause of mortality in affected herds.
Cytokines in Porcine Reproduction
Cytokines influence everything from the initial stages of pregnancy to the final phases of parturition. These signaling molecules are involved in regulating immune responses within the reproductive tract, facilitating communication between maternal and embryonic tissues, and ensuring the proper development of the placenta. Their balanced activity is essential for successful reproduction, with any dysregulation potentially leading to fertility issues or pregnancy complications.
Role in Pregnancy Establishment and Maintenance
During the early stages of pregnancy, cytokines are instrumental in preparing the uterine environment for embryo implantation. Key cytokines such as interleukin-1 (IL-1) and transforming growth factor-beta (TGF-β) are involved in this process. IL-1 is one of the earliest cytokines produced after fertilization, and it plays a pivotal role in modifying the uterine lining (endometrium) to facilitate embryo attachment. It promotes the expression of adhesion molecules and enzymes that remodel the extracellular matrix, making the endometrium receptive to the implanting embryo. TGF-β, on the other hand, is crucial for establishing immune tolerance in the uterus, helping to prevent the maternal immune system from rejecting the semi-allogeneic embryo.
As pregnancy progresses, the development of the placenta—a key organ for nutrient exchange between the mother and fetus—relies heavily on cytokines. Cytokines such as IL-6 and tumor necrosis factor-alpha (TNF-α) are involved in placental angiogenesis, the formation of new blood vessels within the placenta. This process is vital for ensuring that the growing fetus receives an adequate supply of oxygen and nutrients. Additionally, these cytokines help maintain the delicate balance between immune activation and suppression within the placenta, which is essential for protecting the fetus while allowing for appropriate immune surveillance.
Cytokines and Immune Modulation During Pregnancy
Pregnancy is a unique immunological state in which the maternal immune system must tolerate the presence of the fetus while still protecting against infections. Cytokines are central to this immune modulation. During gestation, anti-inflammatory cytokines like interleukin-10 (IL-10) are upregulated to maintain a state of immune tolerance. IL-10 plays a critical role in suppressing potentially harmful inflammatory responses that could lead to fetal rejection or preterm labor. It achieves this by inhibiting the production of pro-inflammatory cytokines and reducing the activity of immune cells that could target the fetal tissues.
Conversely, at the onset of labor, there is a shift in cytokine profiles towards a pro-inflammatory state. Cytokines such as IL-1, IL-6, and TNF-α become more prominent, driving the inflammatory processes that lead to uterine contractions and cervical dilation, which are necessary for the delivery of the piglets. This transition from an anti-inflammatory to a pro-inflammatory environment is tightly regulated, ensuring that labor occurs at the appropriate time.
Implications for Reproductive Success
The balance of cytokine activity is critical for reproductive success in pigs. Disruptions in cytokine signaling can have profound effects on fertility and pregnancy outcomes. For example, insufficient production of pro-inflammatory cytokines during the implantation phase can impair the establishment of pregnancy, leading to early embryonic loss or failure to implant. On the other hand, excessive inflammation during pregnancy can result in complications such as preterm labor, which is a significant cause of neonatal mortality in pigs.
Furthermore, cytokine imbalances can affect litter size and the health of the piglets. High levels of inflammatory cytokines during pregnancy can lead to intrauterine growth restriction (IUGR), where piglets are born smaller and weaker, making them more susceptible to diseases. Conversely, optimal cytokine regulation during gestation supports healthy fetal development, leading to stronger, more viable piglets.
Cytokine Profiles in Sows and Piglets
Throughout pregnancy, cytokine profiles in sows undergo dynamic changes that reflect the evolving needs of the mother and developing piglets. During early gestation, anti-inflammatory cytokines like IL-10 dominate, promoting tolerance and supporting placental development. As gestation progresses, the balance shifts, with a gradual increase in pro-inflammatory cytokines that prepare the sow's body for labor. After birth, the immune challenges of lactation and weaning further influence cytokine production, affecting both the sow and her piglets. The stress associated with weaning, for example, can alter cytokine profiles, leading to immune dysregulation in piglets, which may increase their susceptibility to infections.
Cytokine Modulation through Vaccination and Nutrition
Cytokine modulation through vaccination and nutrition represents a critical approach to enhancing pig health and disease resistance. By understanding how vaccines and dietary components influence cytokine production, researchers and veterinarians can develop more effective strategies for preventing disease and promoting overall well-being in swine populations.
Vaccination Strategies and Cytokine Responses
Vaccination is a cornerstone of disease prevention in pigs, and cytokines play a pivotal role in the immune responses elicited by vaccines. When a pig is vaccinated, the immune system is stimulated to recognize and combat specific pathogens. This process is heavily mediated by cytokines, which help to orchestrate the body's response to the vaccine.
Different types of vaccines can induce varying cytokine profiles. For example, live attenuated vaccines, which contain weakened forms of the pathogen, tend to elicit strong cellular and humoral immune responses. These vaccines often stimulate the production of pro-inflammatory cytokines like interferon-gamma (IFN-γ) and interleukin-12 (IL-12), which are crucial for activating T cells and promoting long-lasting immunity. The robust cytokine response generated by live attenuated vaccines typically leads to more effective protection against the targeted disease. However, the intensity of the response must be carefully managed to avoid excessive inflammation that could harm the pig.
In contrast, inactivated vaccines, which contain killed pathogens, usually induce a more subdued cytokine response. These vaccines often rely on adjuvants—substances added to the vaccine to enhance the immune response—to boost cytokine production. Adjuvants work by stimulating immune cells to produce cytokines that help to activate other parts of the immune system, ensuring that even without a live pathogen, the immune response is strong enough to confer protection. The choice of adjuvant can significantly influence the type and magnitude of cytokine production, thereby shaping the effectiveness of the vaccine. For instance, adjuvants that promote the production of IL-6 and TNF-α can enhance the generation of antibodies, which are crucial for neutralizing pathogens.
Understanding the cytokine profiles induced by different vaccines allows for the design of more targeted vaccination strategies. By selecting the appropriate vaccine type and adjuvant combination, veterinarians can tailor the immune response to the specific needs of the pig, optimizing protection against diseases such as Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) and African Swine Fever Virus (ASFV). Moreover, monitoring cytokine responses following vaccination can provide insights into the efficacy of the vaccine and help in refining vaccination protocols to achieve the best outcomes.
Nutritional Interventions and Cytokine Modulation
Nutrition plays a vital role in the regulation of cytokine production and, by extension, in the modulation of the immune system. The diet of pigs can be strategically manipulated to enhance their immune function and disease resistance, largely through the modulation of cytokine profiles.
Certain dietary components have been identified as key modulators of cytokine production. For instance, omega-3 fatty acids, commonly found in fish oil, have anti-inflammatory properties that can modulate the immune response by reducing the production of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6. This can be particularly beneficial in managing chronic inflammation and improving overall health, especially in pigs that are prone to inflammatory conditions. On the other hand, these fatty acids can also support the production of anti-inflammatory cytokines like IL-10, helping to maintain a balanced immune response that protects against overactive inflammation while still allowing effective pathogen defense.
Vitamins are another crucial aspect of nutritional modulation. Vitamins A, D, and E are known to influence cytokine production. For example, vitamin D has been shown to promote the production of IL-10, an anti-inflammatory cytokine, while simultaneously enhancing the pathogen-fighting capabilities of macrophages through the production of antimicrobial peptides. Similarly, vitamin A is essential for maintaining mucosal immunity, and it modulates cytokine production to support the integrity of epithelial barriers, which are the first line of defense against pathogens. Vitamin E, with its antioxidant properties, helps to protect immune cells from oxidative stress, thereby supporting the overall immune function and influencing cytokine production in favor of a balanced immune response.
Trace minerals such as zinc and selenium also play important roles in cytokine modulation. Zinc is critical for the proper function of immune cells, and its deficiency can lead to impaired cytokine production and weakened immune responses. Selenium, an essential component of antioxidant enzymes, helps to mitigate the oxidative stress that can drive excessive cytokine production, particularly in the context of inflammatory diseases. By ensuring adequate levels of these minerals in the diet, farmers can support the immune system's ability to produce cytokines that are necessary for defending against infections without tipping the balance toward harmful inflammation.
Probiotics and prebiotics represent another nutritional strategy for modulating cytokines. These supplements influence the gut microbiota, which in turn plays a significant role in shaping the immune system's cytokine production, particularly in the gut-associated lymphoid tissue (GALT). Probiotics, which are live beneficial bacteria, can promote the production of anti-inflammatory cytokines like IL-10, helping to maintain gut health and protect against gastrointestinal diseases. Prebiotics, which are non-digestible food ingredients that stimulate the growth of beneficial bacteria, can similarly influence cytokine production, supporting a healthy immune response.
Reference:
- Bleuzé, Marêva, Marcelo Gottschalk, and Mariela Segura. "Neutrophils in Streptococcus suis infection: from host defense to pathology." Microorganisms 9.11 (2021): 2392.

