Introduction to the PI3K-Akt Pathway
The Phosphoinositide 3-kinase (PI3K)-Akt pathway stands as a pivotal signaling cascade that orchestrates a multitude of essential cellular processes. Operating at the intersection of intricate molecular interactions, this pathway plays a paramount role in the regulation of cell survival, growth, metabolism, and more.
At its core, the PI3K-Akt pathway translates external cues, such as growth factors and cytokines, into intricate intracellular responses. By doing so, it exercises intricate control over cell fate and function, contributing profoundly to both physiological and pathological contexts. The pathway consists of a sequence of events whereby activation of PI3K initiates a cascade that culminates in the activation of Akt, also known as Protein Kinase B (PKB).
Molecular Components and Regulation of the PI3K-Akt Pathway
The PI3K-Akt pathway, a master regulator of cell signaling, derives its complexity from a constellation of interconnected molecular components. These components, including Phosphoinositide 3-kinase (PI3K), Akt (Protein Kinase B), and a network of downstream effectors, collaborate to transmit signals that govern fundamental cellular behaviors. Understanding their interactions and regulation provides insight into the intricate machinery driving cell fate determination.
Key Molecular Players
Phosphoinositide 3-kinase (PI3K): This enzyme is a linchpin in the pathway's initiation. Upon ligand-receptor binding, PI3K is recruited to the cell membrane where it phosphorylates phosphatidylinositol lipids, generating phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 serves as a docking site for downstream signaling proteins.
Akt (Protein Kinase B): Akt, a serine/threonine kinase, is activated by binding to PIP3 at the cell membrane. This recruitment leads to Akt's phosphorylation and activation by upstream kinases. Once activated, Akt is translocated to various cellular compartments where it phosphorylates a myriad of downstream substrates, thereby modulating diverse cellular functions.
Downstream Targets and Cellular Responses
Akt's versatility stems from its broad range of downstream targets. These include transcription factors, apoptotic regulators, protein synthesis machinery, and components involved in cell cycle progression. By phosphorylating and regulating these targets, Akt influences essential cellular processes such as:
Cell Survival: Akt phosphorylates and inhibits pro-apoptotic factors, promoting cell survival in adverse conditions.
Cell Growth and Protein Synthesis: Akt activation enhances protein synthesis and cell growth by promoting the activity of mTOR (mammalian target of rapamycin) pathway.
Cell Metabolism: Akt influences glucose metabolism by promoting the translocation of glucose transporters to the cell membrane and modulating enzymes involved in glycolysis.
Initiation and Regulation
The pathway's initiation is tightly controlled by ligand-receptor interactions. Growth factors, cytokines, and hormones activate receptor tyrosine kinases, such as the insulin receptor or epidermal growth factor receptor. This leads to receptor autophosphorylation and recruitment of PI3K to the membrane.
Regulation of Akt Activation
Akt activation is a multi-step process. Phosphorylation at two key sites, Thr308 and Ser473, by phosphoinositide-dependent kinase 1 (PDK1) and mTOR complex 2 (mTORC2), respectively, is essential for full Akt activity. Akt activation can also be dampened by phosphatases that dephosphorylate these sites.
Integration with Other Signaling Pathways
The PI3K-Akt pathway does not function in isolation; it extensively crosstalks with other signaling networks. Notably, it interacts with the Ras-MAPK pathway, which contributes to cell proliferation, and the Wnt pathway, which plays a role in cell fate determination. The interplay between these pathways allows cells to integrate signals from various sources and fine-tune their responses.
Multifaceted Influence of the PI3K-Akt Pathway on Cellular Processes
The PI3K-Akt pathway is a master regulator that orchestrates an array of crucial cellular processes, thereby shaping cell behavior and determining overall cellular fate. Its influence extends across diverse realms, encompassing cell proliferation, apoptosis, protein synthesis, glucose metabolism, and notably, cytokine-driven immune responses. By exerting its control over these processes, the pathway emerges as a pivotal nexus for cellular homeostasis and adaptability.
Cell Proliferation and Growth:
One of the most prominent roles of the PI3K-Akt pathway lies in its regulation of cell proliferation. Akt's activation stimulates cell cycle progression by promoting the transcription of genes involved in cell division and by inhibiting key cell cycle inhibitors. This influence on cell proliferation underscores the pathway's essential contribution to tissue growth, development, and regeneration.
Apoptosis and Cell Survival:
The PI3K-Akt pathway plays a vital role in balancing cell survival and programmed cell death (apoptosis). Akt phosphorylates and inactivates pro-apoptotic proteins, such as Bad, and activates anti-apoptotic proteins like Bcl-2. By doing so, the pathway safeguards cells from apoptosis, ensuring their survival in response to growth factors and favorable conditions.
Protein Synthesis and Cell Growth:
Akt's intricate connection with protein synthesis machinery profoundly impacts cell growth. It stimulates protein translation by phosphorylating and inhibiting TSC2, a negative regulator of mTORC1. This, in turn, activates mTORC1, a central regulator of protein synthesis, leading to increased cell size and mass.
Glucose Metabolism:
The PI3K-Akt pathway is a key player in glucose homeostasis. Akt activation enhances glucose uptake by translocating glucose transporters (GLUT4) to the cell membrane. Additionally, Akt regulates enzymes involved in glycolysis and glycogen synthesis, facilitating energy production and storage.
Cytokine Signaling and Immune Responses:
The pathway's influence on immune responses is particularly noteworthy. Cytokines, which serve as crucial mediators of immune signaling, can activate the PI3K-Akt pathway. Conversely, Akt activation can influence cytokine production and secretion by immune cells. The pathway's involvement in immune cell survival, proliferation, and cytokine production highlights its role in shaping immune responses. Akt's impact on the development and function of various immune cell types, including T cells, B cells, and macrophages, underscores its significance in modulating immune system behavior.
Integration of Cytokine and Growth Factor Signaling:
Furthermore, the PI3K-Akt pathway bridges cytokine signaling and growth factor responses. Some cytokines, such as interleukins, tumor necrosis factor (TNF), and interferons, can activate the PI3K-Akt pathway, promoting various immune responses. This integration allows cells to coordinate their reactions to both external stimuli and internal growth signals, ensuring a comprehensive and adaptive cellular response.
Dysregulation of the PI3K-Akt Pathway
The PI3K-Akt pathway's pivotal role in governing diverse cellular processes also renders it a key player in the development and progression of various diseases. Dysregulation of this pathway has been implicated in a spectrum of disorders, ranging from cancer to metabolic disorders and inflammatory conditions.
Cancer and Tumor Progression
Perhaps the most well-studied association, aberrant activation of the PI3K-Akt pathway is a hallmark of many cancer types. Mutations or amplifications in genes encoding components of the pathway, such as PI3K, Akt, or PTEN (Phosphatase and Tensin Homolog), lead to persistent pathway activation. This results in uncontrolled cell proliferation, resistance to apoptosis, and enhanced angiogenesis—key features of tumor growth and progression. Consequently, targeted therapies aiming to inhibit PI3K-Akt signaling have garnered significant attention in cancer treatment.
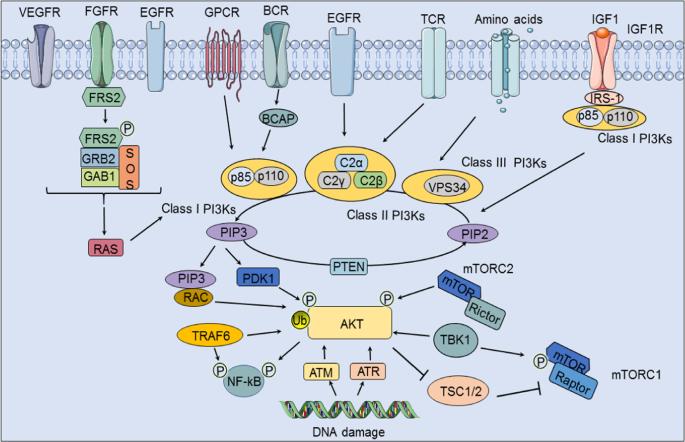 Upstream
activation of the PI3K/Akt signaling pathway (He et al., 2021).
Upstream
activation of the PI3K/Akt signaling pathway (He et al., 2021).
Metabolic Disorders and Diabetes
The PI3K-Akt pathway's involvement in glucose metabolism renders it crucial in metabolic disorders like type 2 diabetes. Insulin resistance, a hallmark of the disease, often involves impaired insulin signaling through the PI3K-Akt pathway. Dysfunctional pathway components contribute to reduced glucose uptake and compromised glycogen synthesis in target tissues, culminating in hyperglycemia. Investigating the intricacies of this pathway in metabolic disorders offers potential avenues for novel therapeutic interventions.
Inflammatory Conditions and Immune Dysfunction
The PI3K-Akt pathway's influence on immune responses also makes it a key player in inflammatory conditions. Overactivation of the pathway in immune cells can lead to heightened inflammatory responses, contributing to chronic inflammatory diseases like rheumatoid arthritis and inflammatory bowel disease. Conversely, impaired pathway activity can compromise immune cell survival and function, leading to immunodeficiency disorders. Modulating the PI3K-Akt pathway presents an opportunity to fine-tune immune responses for therapeutic benefit.
Therapeutic Implications and Drug Development
Understanding the intricacies of the PI3K-Akt pathway's dysregulation in disease provides a foundation for targeted therapeutic strategies. Pharmacological interventions designed to inhibit or modulate specific pathway components hold promise for precision medicine approaches. Small molecule inhibitors targeting PI3K or Akt isoforms have been developed and are being evaluated in clinical trials for various diseases, including cancer. Additionally, combination therapies that integrate PI3K-Akt pathway inhibition with other targeted agents or immunotherapies are being explored to enhance treatment efficacy.
Interplay between Cytokine Signaling and the PI3K-Akt Pathway
The intricate web of cellular signaling extends beyond individual pathways, intertwining to orchestrate complex cellular responses. The crossroads of cytokine signaling and the PI3K-Akt pathway exemplify such interplay, with cytokines influencing the pathway and vice versa. This dynamic relationship holds significance in modulating immune responses, cellular behaviors, and disease processes.
Cytokines and PI3K-Akt Pathway Activation
Cytokines, small proteins pivotal in cell-to-cell communication, can initiate signals that activate the PI3K-Akt pathway. Receptors for certain cytokines, such as interleukins (IL) and growth factors, trigger intracellular signaling cascades through adaptor proteins that interact with PI3K. For example, IL-3 and IL-7 activate the PI3K-Akt pathway by promoting the recruitment of PI3K to their respective receptors.
Modulation of Cytokine Production by PI3K-Akt Pathway
Conversely, the PI3K-Akt pathway can impact cytokine production. Upon activation, Akt can phosphorylate transcription factors, such as NF-κB and STAT3, that govern cytokine gene expression. This phosphorylation enhances the transcriptional activity of these factors, influencing the production of cytokines like IL-2, IL-6, and TNF-α.
Examples of Cytokines Influencing the Pathway
- Interleukin-6 (IL-6): This pleiotropic cytokine is a potent activator of the PI3K-Akt pathway. IL-6 receptor engagement triggers intracellular signaling cascades that culminate in Akt activation. IL-6-induced Akt activation contributes to various cellular processes, including cell survival and proliferation.
- Tumor Necrosis Factor-alpha (TNF-α): TNF-α, a pro-inflammatory cytokine, can activate the PI3K-Akt pathway. This activation can promote cell survival and proliferation, contributing to inflammation-related cellular responses.
- Interleukin-3 (IL-3): IL-3, a hematopoietic growth factor, can stimulate the PI3K-Akt pathway in hematopoietic cells. This activation supports cell survival and proliferation, crucial for the expansion and maintenance of various blood cell populations.
Reciprocal Regulation: A Feedback Loop
This reciprocal regulation forms a feedback loop that influences cellular behaviors and immune responses. Cytokines can enhance Akt activation, which in turn can influence cytokine production. This crosstalk enables cells to integrate external cues with their intracellular state, facilitating a balanced and context-dependent response to environmental challenges.
Select Service
Illuminating the PI3K-Akt Pathway and Cytokine Interactions with Luminex xMAP Technology
Unlocking the intricate interplay between the PI3K-Akt pathway and cytokine signaling is essential for advancing scientific understanding and medical breakthroughs. Our specialized cytokine analysis services, powered by Luminex xMAP technology, offer a cutting-edge platform to researchers seeking to unravel the complexities of these dynamic interactions.
Our team comprises experts in cell signaling, immunology, and molecular biology, with a deep understanding of the PI3K-Akt pathway and cytokine networks. Leveraging the power of Luminex xMAP technology, we provide comprehensive and precise analyses, ensuring high-quality data that drive insightful conclusions.
Our services enable detailed investigation of the PI3K-Akt pathway's activation and modulation. Through advanced techniques, we quantify key phosphorylation events and activity levels of pathway components in response to various stimuli, shedding light on its role in cell survival, growth, and disease.
The integration of Luminex xMAP technology allows us to profile cytokine responses in the context of the PI3K-Akt pathway with exceptional precision. By analyzing cytokine production, secretion, and downstream effects, we unveil how the pathway influences immune responses and cellular behaviors.
Reference:
- He, Yan, et al. "Targeting PI3K/Akt signal transduction for cancer therapy." Signal transduction and targeted therapy 6.1 (2021): 425.