Introduction to the NOD-like Receptor (NLR) Pathway
The NOD-like receptor (NLR) pathway stands as a pivotal component of the innate immune system, orchestrating the body's defense against invading pathogenic microorganisms. NLRs, a family of intracellular receptors, emerge as sentinels, surveying the cellular environment for the presence of dangerous pathogens and swiftly initiating a cascade of immune responses to counteract potential threats.
At the heart of this intricate defense mechanism lies the remarkable ability of NLRs to distinguish between self and non-self entities. This capacity is imperative for safeguarding the body against microbial invaders while preserving the integrity of healthy tissues. NLRs are renowned for their role in surveilling the cytoplasmic compartment of cells, where they stand ready to trigger immune reactions upon detecting conserved microbial molecules or signs of cellular stress.
NLRs exhibit a modular structure comprised of distinct domains, each bearing a specific function. The N-terminal effector-binding domain serves as the initial point of interaction, where NLRs engage with various ligands, including pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). This interaction sets the stage for downstream events that culminate in the activation of immune responses.
Central to the NLR structure is the nucleotide-binding and oligomerization domain, known as the NACHT domain. This domain serves as a molecular switch, undergoing conformational changes upon ligand binding. These changes trigger the assembly of large protein complexes, termed inflammasomes, which are central to initiating inflammation and cytokine production. The C-terminal leucine-rich repeat (LRR) domain adds an additional layer of specificity, fine-tuning the recognition of distinct ligands and pathogens.
NLRs play a multifaceted role in recognizing a wide array of pathogens, ranging from bacteria and viruses to fungi and parasites. Upon activation, NLRs can engage various downstream signaling pathways, ultimately leading to the production of pro-inflammatory cytokines and interferons. This orchestration of immune responses serves to contain the infection, recruit immune cells, and stimulate adaptive immunity for long-term defense.
Structure and Types of NLRs
The structural organization of NOD-like receptors (NLRs) underpins their diverse functions in pathogen recognition and immune activation. NLRs consist of distinct domains that collectively enable them to engage with various ligands and orchestrate immune responses. Three key domains define the architecture of NLRs: the N-terminal effector-binding domain, the central nucleotide-binding and oligomerization domain (NACHT domain), and the C-terminal leucine-rich repeat (LRR) domain.
- N-terminal Effector-Binding Domain
The journey of NLR activation begins at the N-terminal effector-binding domain. This domain serves as the receptor's "eyes and ears," recognizing pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) within the cellular environment. By binding to these ligands, NLRs initiate a conformational change that sets off a cascade of events leading to immune responses.
- Nucleotide-binding and Oligomerization Domain (NACHT)
At the core of the NLR structure lies the NACHT domain. This domain functions as a nucleotide-binding and oligomerization platform. Upon ligand binding, the NACHT domain undergoes dynamic conformational changes and self-oligomerization. These structural alterations drive the assembly of large protein complexes known as inflammasomes. The NACHT domain is not only central to signal transduction but also to the formation of higher-order complexes that amplify immune signaling.
- C-terminal Leucine-rich Repeat (LRR) Domain
The C-terminal LRR domain contributes a critical layer of specificity to NLR function. Comprising a series of repeated motifs, the LRR domain serves as a versatile recognition module. This module fine-tunes ligand specificity, enabling NLRs to differentiate between various pathogens and danger signals. The LRR domain also participates in mediating protein-protein interactions within the larger inflammasome complex.
NLR Subfamilies and Functions
The vast diversity of NLRs is reflected in distinct subfamilies, each tailored to recognize specific types of microbial threats. Three prominent subfamilies include NLRP (NACHT, LRR, and pyrin domain-containing), NLRC (NACHT, LRR, and CARD domain-containing), and NLRB (NACHT, LRR, and B30.2/SPRY domain-containing). Each subfamily is characterized by the presence of unique domains that contribute to their specialized functions:
- NLRP Subfamily: NLRP proteins are pivotal components of inflammasomes, responsible for activating caspase-1 and promoting the maturation and release of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18). This subfamily plays a central role in initiating and sustaining immune responses against infections and tissue damage.
- NLRC Subfamily: NLRC proteins often possess CARD (caspase activation and recruitment domain) domains, which facilitate their interactions with downstream signaling molecules. NLRC proteins contribute to a broader range of immune responses beyond inflammasome activation, including the regulation of NF-κB signaling and interferon responses.
- NLRB Subfamily: NLRB proteins possess B30.2/SPRY domains, which are implicated in protein-protein interactions and ligand recognition. While less characterized compared to NLRP and NLRC subfamilies, NLRB proteins are emerging as modulators of immune responses through interactions with various cellular components.
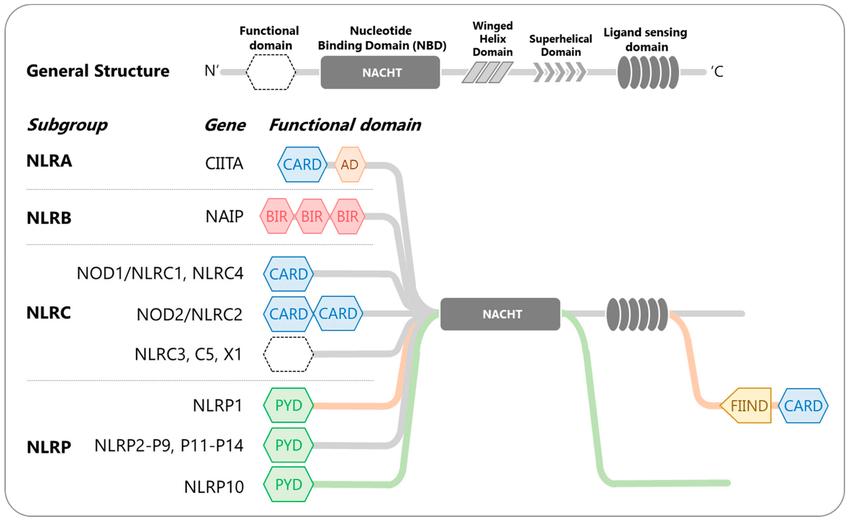 Human NOD-like receptors (NLRs)-classification and domain organization (Lim et al., 2020).
Human NOD-like receptors (NLRs)-classification and domain organization (Lim et al., 2020).
NLR Activation Mechanisms and Inflammasome Formation
Activation of NOD-like receptors (NLRs) is a sophisticated process that involves intricate molecular interactions and signaling events. NLRs serve as guardians of the cellular environment, capable of detecting both pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), which signal the presence of microbial invaders or cellular stress.
1. Detection of PAMPs and DAMPs:
NLR activation begins with the recognition of PAMPs and DAMPs. PAMPs are conserved molecular patterns found on the surface of pathogens, such as bacterial cell wall components or viral nucleic acids. DAMPs, on the other hand, are molecules released by stressed or damaged cells, signaling the presence of danger or tissue injury. These patterns are recognized by the N-terminal effector-binding domain of NLRs, initiating a conformational change that primes the receptor for activation.
2. Oligomerization of NACHT Domain:
Upon ligand binding, NLRs undergo a conformational change in their NACHT domain, leading to the oligomerization of NLRs. This self-assembly results in the formation of large protein complexes called inflammasomes. Inflammasomes serve as platforms for the recruitment and activation of downstream signaling molecules, most notably caspase-1.
3. Inflammasome Assembly and Caspase Activation:
Inflammasomes are multiprotein complexes comprised of NLRs, the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), and pro-caspase-1. ASC links NLRs to pro-caspase-1, promoting its autocatalytic cleavage and activation into caspase-1. Activated caspase-1 then processes pro-inflammatory cytokines, such as pro-IL-1β and pro-IL-18, into their mature forms, which can be released into the extracellular space.
4. Cytokine Production and Inflammation:
The cleavage and release of mature IL-1β and IL-18 by activated caspase-1 are central to the initiation of inflammation. These cytokines exert potent pro-inflammatory effects, attracting immune cells to the site of infection or tissue damage. This localized immune response aids in neutralizing pathogens, promoting tissue repair, and ultimately reestablishing homeostasis.
5. Pyroptosis and Cellular Defense:
In addition to cytokine production, inflammasome activation can also trigger a form of programmed cell death known as pyroptosis. Pyroptosis is characterized by cell swelling, membrane rupture, and the release of pro-inflammatory cellular contents. This process eliminates the infected cell while alerting neighboring cells to the presence of danger, contributing to the containment of infections.
The concept of inflammasomes highlights the sophisticated nature of NLR activation and its crucial role in orchestrating immune responses. The ability of NLRs to distinguish between PAMPs and DAMPs ensures that immune reactions are tailored to the specific threat at hand. This remarkable capacity for discernment enables the immune system to effectively combat infections while minimizing collateral damage to healthy tissues. The intricate interplay between NLRs, inflammasomes, and downstream cytokine production serves as a testament to the immune system's intricate and dynamic defense mechanisms.
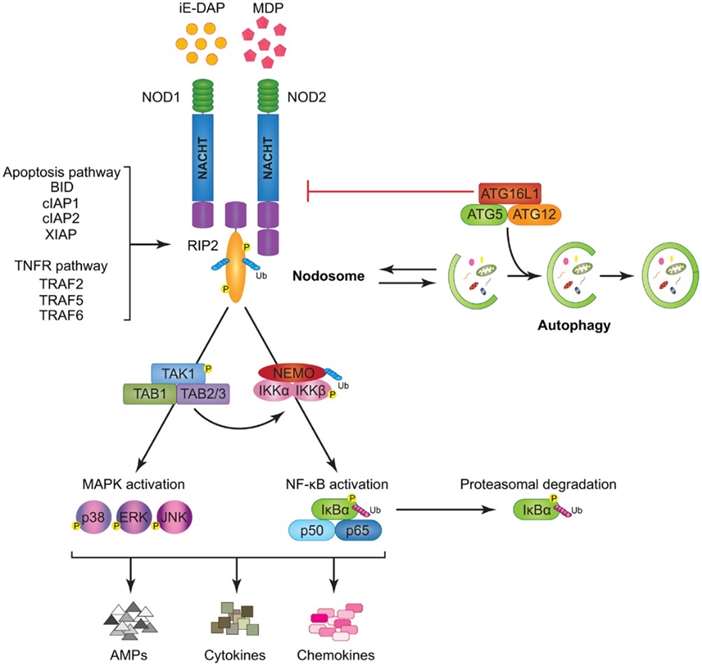 Modeling of nod1
and nod2 signaling cascades (Saxena et al., 2014).
Modeling of nod1
and nod2 signaling cascades (Saxena et al., 2014).
Role of the NLR Pathway in Cytokine Regulation and Immune Homeostasis
The NOD-like receptor (NLR) pathway stands as a pivotal regulator of cytokine production within the immune system, orchestrating a dynamic interplay of molecular events that shape immune responses and maintain immune homeostasis.
1. IL-1β and IL-18 Regulation:
The activation of certain NLRs, notably those belonging to the NLRP subfamily, drives the production of pro-inflammatory cytokines IL-1β and IL-18. Upon recognition of microbial ligands or danger signals, NLRP-containing inflammasomes assemble and activate caspase-1, which processes pro-IL-1β and pro-IL-18 into their mature forms. These cytokines are then released into the extracellular space, where they exert potent effects on immune responses.
2. Immune Response Amplification:
IL-1β and IL-18 function as critical mediators that amplify immune responses. IL-1β stimulates the recruitment of immune cells to sites of infection or injury, enhances phagocytosis, and facilitates the adaptive immune response by promoting T cell activation. IL-18, on the other hand, augments the production of interferon-gamma (IFN-γ), which enhances the antimicrobial activity of immune cells. The NLR pathway's role in regulating these cytokines ensures an appropriate and timely immune response to microbial threats.
3. Immune Homeostasis:
Beyond acute immune responses, the NLR pathway also contributes to immune homeostasis. NLRs are involved in regulating immune cell proliferation, differentiation, and survival. By fine-tuning cytokine production, NLRs help to prevent excessive inflammation that can lead to tissue damage and autoimmune disorders. This delicate balance ensures that immune responses are effective yet controlled, maintaining the body's equilibrium.
4. Adaptive Immunity Linkage:
The activation of the NLR pathway not only influences innate immune responses but also bridges with adaptive immunity. IL-1β and IL-18 act as "alarmins," signaling to dendritic cells and other antigen-presenting cells to enhance antigen presentation to T cells. This communication ensures the coordination of innate and adaptive immune responses, fostering a comprehensive defense strategy against pathogens.
5. Dysregulation and Disease Implications:
Dysregulation of the NLR pathway and its associated cytokine production can lead to various diseases. Excessive IL-1β production, for instance, is linked to autoinflammatory disorders, while altered IL-18 levels are implicated in autoimmune diseases. Understanding the intricate mechanisms of NLR activation and cytokine regulation is crucial for developing targeted therapies to correct these imbalances and restore immune homeostasis.
Select Service
NLR Pathway Dysregulation in Diseases and Research
Autoimmune Disorders
Dysregulation of the NLR pathway can contribute to autoimmune disorders, where the immune system mistakenly attacks healthy tissues. For instance, mutations in the NLRP3 gene are associated with autoinflammatory diseases like cryopyrin-associated periodic syndromes (CAPS), characterized by excessive IL-1β production and chronic inflammation. Studying these mutations has deepened our understanding of IL-1β's role in autoimmunity and has led to the development of targeted IL-1β inhibitors as therapies.
Inflammatory Conditions
Inflammatory conditions, such as inflammatory bowel diseases (IBD), have been linked to NLR pathway dysregulation. NLRs play a role in maintaining gut homeostasis, and their malfunction can lead to uncontrolled inflammation in the intestines. Studying the NLRP6 inflammasome has revealed insights into its role in regulating gut microbial composition and maintaining gut barrier integrity, highlighting its potential as a therapeutic target for IBD.
Infectious Diseases
NLRs are critical in defending against infectious agents. Dysregulation can both compromise immunity and exacerbate inflammation. In tuberculosis, for example, NLRP3 inflammasome activation is implicated in the pathogenesis of granulomas, which contribute to tissue damage. Targeting NLRs or their downstream pathways could offer novel ways to modulate immune responses and enhance host defense against infections.
Cancer Immunity
Emerging evidence suggests that NLRs might also impact the immune response against cancer. NLRP3 inflammasome activation has been shown to play a role in antitumor immunity. Enhancing NLR-mediated immune responses could potentially bolster the body's ability to recognize and eliminate cancer cells, opening avenues for immunotherapeutic strategies.
Drug Discovery
The NLR pathway offers promising targets for drug discovery. By identifying molecules that modulate NLR activation or downstream signaling, researchers can develop innovative treatments for a wide range of diseases characterized by immune dysregulation.
Luminex Cytokine Analysis in NLR Pathway Research
Luminex cytokine analysis has emerged as a transformative tool for unraveling the intricate connections between the NOD-like receptor (NLR) pathway and cytokine responses. This technology enables researchers to comprehensively explore how NLR activation shapes immune reactions. By simultaneously quantifying a diverse array of cytokines within a single sample, Luminex analysis provides a panoramic view of the immune landscape influenced by the NLR pathway.
Researchers harness the power of Luminex cytokine analysis to uncover specific cytokine patterns triggered by NLR activation. Whether examining responses to NLR ligands or contrasting healthy and disease-affected samples, this approach offers a nuanced understanding of cytokine dynamics driven by NLR signaling. These insights help illuminate disease mechanisms and provide crucial information about immune responses to infection, inflammation, and other pathophysiological processes.
Furthermore, the versatility of Luminex technology extends to unveiling intricate cytokine networks orchestrated by the NLR pathway. By evaluating correlations and co-expression patterns among various cytokines, researchers gain a holistic perspective on how NLR activation orchestrates immune regulation. This knowledge deepens our understanding of immune homeostasis and informs potential interventions for diseases marked by NLR dysfunction.
Luminex cytokine analysis also offers a gateway to biomarker discovery. By comparing cytokine profiles between healthy and diseased individuals, researchers can identify cytokines that serve as potential biomarkers for diagnosing, prognosing, or monitoring diseases influenced by NLR dysregulation.
References:
- Saxena, Mansi, and Garabet Yeretssian. "NOD-like receptors: master regulators of inflammation and cancer." Frontiers in immunology 5 (2014): 327.
- Lim, Rayne R., et al. "NOD-like receptors in the eye: uncovering its role in diabetic retinopathy." International Journal of Molecular Sciences 21.3 (2020): 899.