Luminex multiplex cytokine assays have become essential instruments in the fields of immunology, biomarker exploration, and clinical diagnostics. These assays enable researchers to concurrently assess numerous cytokines within a single sample, yielding extensive understanding of immune responses, inflammation, and disease pathways. To effectively utilize Luminex technology, it is imperative to grasp and establish three vital parameters: the Lower Limit of Detection (LLD), the Lower Limit of Quantification (LLOQ), and the Upper Limit of Quantification (ULOQ).
Select Service
LLD (Lower Limit of Detection)
LLD in Luminex multiplex cytokine assays refers to the minimum concentration of a cytokine or biomarker that can be reliably distinguished from background noise and accurately quantified using the Luminex multiplex technology. This parameter is of paramount importance in scientific research and clinical diagnostics, as it defines the assay's sensitivity and its ability to detect low levels of analytes in biological samples.
Several key points should be considered when discussing LLD in Luminex assays:
Principle of Detection: Luminex technology relies on fluorescent microspheres, each coated with specific capture antibodies to bind target cytokines in a sample. These microspheres are tagged with distinct fluorophores, and the fluorescence signal generated upon target binding is measured. LLD is determined by the level of fluorescence that can be reliably distinguished from the background signal.
Signal-to-Noise Ratio: The LLD is essentially the concentration at which the signal (specific cytokine detection) is significantly higher than the background noise. It is typically defined as a signal-to-noise ratio of 2:1 or 3:1, indicating that the signal is at least two or three times greater than the noise level.
Validation and Calibration: To establish the LLD, rigorous validation and calibration procedures are conducted. This involves analyzing known concentrations of the cytokine of interest in multiple replicates to generate a standard curve. The LLD is then determined as the lowest concentration on the standard curve that consistently yields a signal above the noise threshold.
Clinical Significance: Understanding the LLD is critical when interpreting experimental or clinical results. Concentrations below the LLD may not be reliable and could lead to erroneous conclusions regarding the presence or absence of specific cytokines in the sample.
Sample Matrix Effects: It's important to note that the LLD can vary depending on the nature of the biological sample being analyzed. Complex sample matrices, such as serum or plasma, may introduce additional challenges in achieving a low LLD due to interference from other components. Sample pre-treatment and assay optimization are often necessary to mitigate these effects.
LLOQ (Lower Limit of Quantification)
Lower Limit of Quantification (LLOQ) represents the lowest concentration of a cytokine that can be accurately and reliably quantified with a defined level of precision and accuracy. LLOQ serves as a crucial demarcation point in these assays, marking the transition from qualitative detection to quantitative measurement.
The role of LLOQ in multiplex cytokine detection is multifaceted and underpins the validity of quantitative results. It sets the standard for the lower boundary of quantitative measurement, ensuring that values obtained above this threshold are not only detectable but also possess the necessary precision to make meaningful comparisons between samples. In essence, LLOQ defines the dynamic range of the assay, delineating the lower limit of reliable quantification.
Determining LLOQ is a meticulous process that involves constructing a standard curve using a series of known cytokine concentrations, including concentrations near the expected LLOQ. This curve serves as a reference for quantifying cytokine levels in test samples. The goal is to establish a linear, accurate, and precise relationship between cytokine concentration and assay response within the LLOQ range. The LLOQ is typically calculated as the lowest concentration at which the coefficient of variation (CV) falls below a predefined threshold, indicating acceptable precision.
Implementing LLOQ in practice requires strict adherence to established protocols and quality control measures. Maintaining the assay's accuracy and precision over time necessitates routine validation and ongoing verification of LLOQ. This ensures that the assay remains within its validated performance specifications, enabling researchers and clinicians to consistently generate reliable quantitative cytokine data.
ULOQ (Upper Limit of Quantification)
The Upper Limit of Quantification (ULOQ) stands as a pivotal parameter in Luminex multiplex cytokine assays, holding significant importance within the realms of immunology and biomarker research. ULOQ signifies the highest concentration of a cytokine or analyte within a specific assay that can be accurately and precisely quantified. This parameter serves as a crucial boundary, defining the upper limit of the dynamic range for analyte quantification, thereby ensuring the meaningfulness and reliability of measurements.
The determination of ULOQ is a meticulous process that involves extensive assay optimization and validation. Here is a detailed explanation of the factors and steps involved in establishing ULOQ in Luminex multiplex cytokine assays:
Selection of Calibration Standards: The first step is to choose a set of calibration standards that span the expected range of analyte concentrations in the sample. These standards should ideally cover several orders of magnitude to ensure accuracy across the entire dynamic range of the assay.
Curve Fitting: A calibration curve is generated by plotting the mean fluorescent intensity (MFI) or signal response against the known concentrations of the calibration standards. Curve fitting algorithms, such as 4-parameter logistic (4PL) models, are applied to this data to establish a mathematical relationship between signal and concentration.
Determination of Limit of Detection (LOD): LOD represents the lowest concentration at which the analyte can be reliably detected but not necessarily quantified. It is typically calculated based on background signal levels and is an important parameter to distinguish signal from noise.
Evaluation of Precision: Precision studies are conducted to assess the repeatability and reproducibility of the assay at various concentrations, including the ULOQ. This involves running replicates of the same samples to calculate coefficients of variation (CV) and ensure that the measurements are precise.
Accuracy Assessment: Accuracy is evaluated by comparing the measured concentrations of the calibration standards to their known concentrations. This step ensures that the assay provides unbiased and reliable quantification, especially at the ULOQ.
Dilution Linearity: Dilution studies are performed to assess the linearity of the assay at concentrations near the ULOQ. This helps confirm that diluting samples within the dynamic range maintains accuracy and precision.
Matrix Effects: In multiplex assays, samples may contain various matrices (e.g., serum, plasma) that can influence the assay's performance. Matrix effects, especially at the ULOQ, need to be evaluated and addressed.
Data Analysis Software: Utilization of specialized software for data analysis is common, as it aids in accurately determining ULOQ and other assay parameters, taking into account curve fitting, background correction, and statistical analysis.
Validation and Documentation: The entire ULOQ determination process is meticulously validated and documented. This documentation is crucial for ensuring the assay's reliability and reproducibility in future experiments.
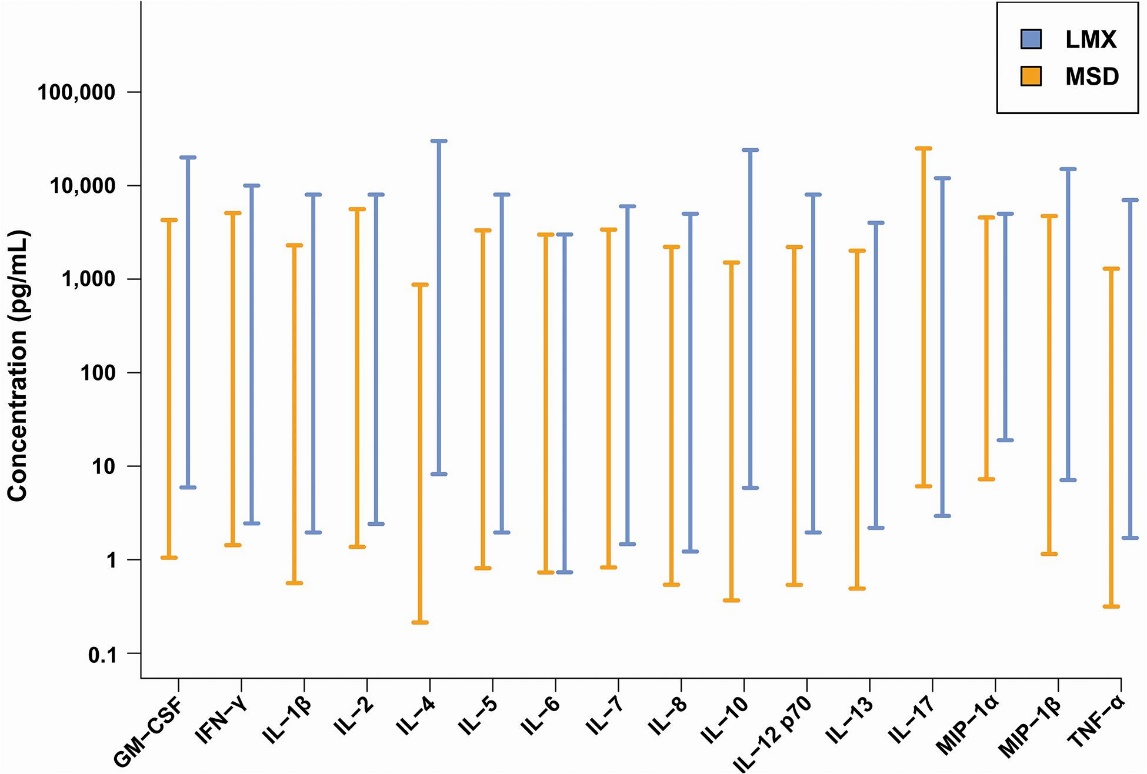 Dynamic ranges of the Luminex bead-based fluorescence (LMX) and Meso Scale Discovery electrochemiluminescence (MSD) multiplex cytokine immunoassay (Günther et al., 2020)
Dynamic ranges of the Luminex bead-based fluorescence (LMX) and Meso Scale Discovery electrochemiluminescence (MSD) multiplex cytokine immunoassay (Günther et al., 2020)
Practical Considerations
Validation: Laboratories conducting Luminex multiplex cytokine assays should establish LLD, LLOQ, and ULOQ values during assay validation. This rigorous validation process ensures the reliability and reproducibility of assay results.
Sample Dilution: For samples with cytokine concentrations near or above the ULOQ, appropriate sample dilution should be performed to bring the concentrations within the quantifiable range of the assay.
Quality Control: Regular quality control measures, such as using control samples with known concentrations, should be implemented to monitor assay performance and verify that LLD, LLOQ, and ULOQ criteria are consistently met.
Reference:
- Günther, Anna, et al. "Comparison of bead-based fluorescence versus planar electrochemiluminescence multiplex immunoassays for measuring cytokines in human plasma." Frontiers in immunology 11 (2020): 572634.