Relationship Between Immunity, Inflammation and Cancer
Immunity is an act of self-protection of the body. The immune response is divided into specific and non-specific. The antigen and antibody responses are generally referred to as specific immune responses, which require the participation of B cells and T cells. Other immune cells such as macrophages and NK cells mediate non-specific immune responses, which are part of the body's natural defense system. A proper immune response can clear pathogens and is beneficial to the organism. However, an excessive immune response can be harmful to the organism itself.
The inflammatory response is an important active defense mechanism of the body, but chronic inflammation is one of the predisposing factors for cancer development. The main cellular signaling pathways of immunity and inflammation include:
- Jak/Stat signaling pathways
- NF-κB signaling pathways
- Wnt signaling pathways
- Toll-like recptor siganling pathways
- B cell receptor signaling pathways
- T cell receptor signaling pathways
And cancer development is closely related to related cell signaling pathways. Taking the most used Jak/Stat and NF-kB as examples.
Jak/Stat pathway and cancer
JAK activating mutations are a major molecular mechanism in malignant hematologic diseases. Researchers have identified a unique somatic mutation (V617F) in the Jak2 pseudokinase domain. This mutation, which often occurs in patients with true erythroblastosis, primary thrombocytosis and myelofibrosis, leads to pathological activation of Jak2, which also activates receptors for erythropoietin (EPO), thrombopoietin (TPO) and G-CSF that control the proliferation and differentiation of erythrocytes, megakaryocytes and granulocytes. The functionally acquired somatic mutation of Jak1 has been found in adult acute lymphoblastic leukemia.
Somatic activating mutations have been shown to be present in pediatric acute lymphoblastic leukemia (ALL) patients. In addition, mutations near the Jak2 pseudokinase domain R683 (R683G or ΔIREED) have been identified in children with Down syndrome B-ALL as well as in patients with pediatric Down syndrome.
NF-κB pathway and cancer
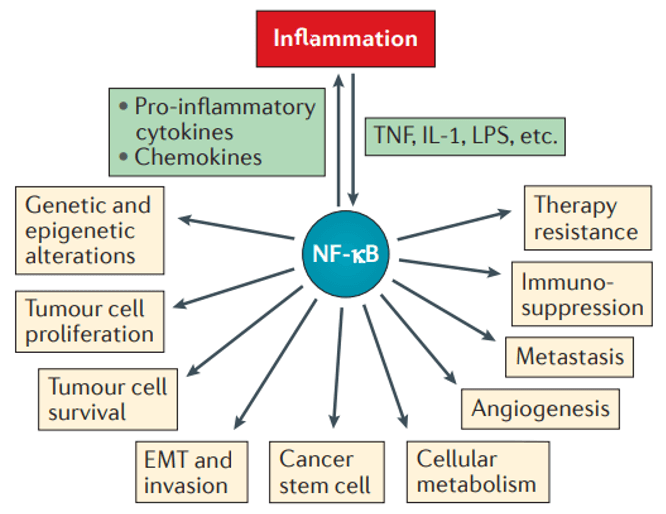 Roles of NF-κB in cancer (Taniguchi et al., 2018)
Roles of NF-κB in cancer (Taniguchi et al., 2018)
A second frequently studied inflammatory pathway is the NF-κB pathway, which is also inextricably linked to cancer. Evidence that NF-κB can transform cells originally came from the discovery of the v-Rel gene from an avian retrovirus, which encodes a protein that is highly oncogenic and can cause malignant cancers in chickens. Later studies revealed that the protein encoded by v-Rel belongs to the Rel subfamily of NF-κB, and several other oncogenic viruses that have been discovered one after another are also oncogenic through activation of NF-κB.
Transcription of genes such as cyclin D1, cyclin-dependent kinase Cdk2, and c-myc is regulated by NF-κB. In cancers such as breast, colon, and lymphoma, persistent activation of NF-κB leads to abnormal activity of cell cycle-related proteins.
In addition, persistent activation of NF-κB increases the levels of a number of cytokines that promote cancer growth, such as IL-1β (acute leukemia growth factor), TNF (malignant lymphogranuloma, T-cell lymphoma, and glioma growth factor), and IL-6 (growth factor in multiple myeloma).
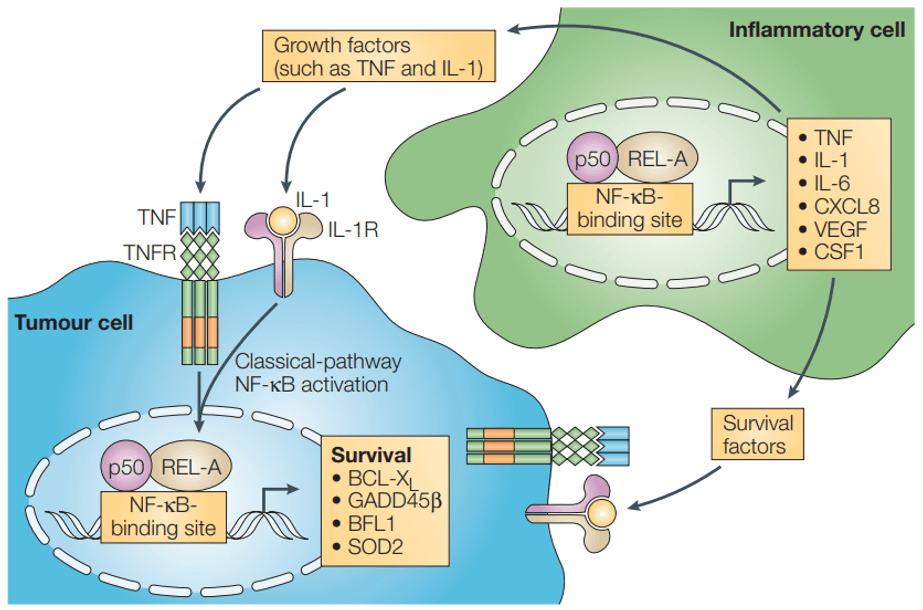 Nuclear factor-κB has various tumour-promoting functions depending on the cell type in which it is activated (Karin et al., 2005)
Nuclear factor-κB has various tumour-promoting functions depending on the cell type in which it is activated (Karin et al., 2005)
Two important stages of cancer development are tissue infiltration and metastasis, both of which are also regulated by NF-κB-dependent genes, including matrix metalloproteinases, urokinase fibrinolytic element activator, and interleukin IL-8. In addition, cancer cell growth and tissue infiltration require constant neointima formation, and genes for proteins associated with mediating neointima production are regulated by NF-κB.
Persistent activation of NF-κB can also lead to anti-apoptotic cell death and resistance to the apoptosis-inducing effects of chemotherapeutic agents.
The above several NF-κB-mediated cancer formation mechanisms can coordinate with each other and interact at different signaling levels to jointly promote tumorigenesis and progression. Currently, tumor-targeted therapy targeting NF-κB is one of the hot spots in cancer treatment.
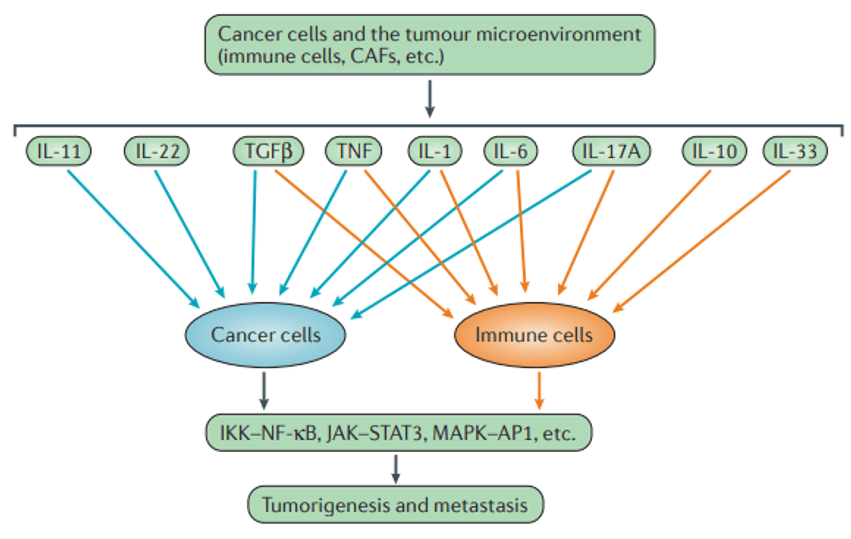 Mode of action of cytokines on cancer and immune cells (Taniguchi et al., 2018).
Mode of action of cytokines on cancer and immune cells (Taniguchi et al., 2018).
Detection of inflammatory factors
Cytokines have the ability to promote and suppress inflammatory responses, so it is important to understand the complex network of secreted cytokines.
Cytokines do not operate independently, but are in a network of activation and inhibition. Assessing the inflammatory response by detecting individual cytokines often does not reflect the complexity of inflammation. Cytokines do not operate independently, but are in a network of activation and inhibition. Assessing the inflammatory response by detecting individual cytokines often does not reflect the complexity of inflammation.
Based on Luminex multiplex technology, Creative Proteomics has developed multiple inflammation panels to measure inflammatory biomarkers. Simultaneous quantification of multiple soluble immune biomarkers using a single sample allows for customized detection and quantification of inflammatory factors.
References:
- Taniguchi, K., & Karin, M. (2018). NF-κB, inflammation, immunity and cancer: coming of age. Nature Reviews Immunology, 18(5), 309-324.
- Singh, R., Mishra, M. K., & Aggarwal, H. (2017). Inflammation, immunity, and cancer. Mediators of inflammation, 2017.
- Karin, M., & Greten, F. R. (2005). NF-κB: linking inflammation and immunity to cancer development and progression. Nature reviews immunology, 5(10), 749-759.

