The IL-17 signaling pathway is a fundamental mechanism within the immune system, exerting a significant influence on immune responses and overall immune equilibrium. At the heart of this pathway is Interleukin-17 (IL-17), a pro-inflammatory cytokine mainly produced by specialized T-helper cells called Th17 cells, as well as other immune cell types like γδ T cells and innate lymphoid cells.
The IL-17 signaling pathway's importance in the context of cytokine analysis is profound, owing to its central role in various physiological and pathological processes. This pathway not only acts as a potent defender against infections but also wields a dual-edged sword in the development of autoimmune diseases and chronic inflammatory conditions.
Comprehending the nuances of the IL-17 signaling pathway holds vital implications for the study of cytokine-related ailments and phenomena. The disruption of IL-17 signaling has been associated with a range of disorders, including autoimmune conditions like psoriasis, rheumatoid arthritis, and inflammatory bowel disease. Furthermore, its participation in bolstering the body's defenses against bacterial and fungal infections underscores its scientific importance. By dissecting the components and intricacies of this pathway, scientists can gain valuable insights into the molecular mechanisms governing immune responses and inflammatory reactions.
Key Components of IL-17 Signaling
The IL-17 signaling pathway operates through a sophisticated interplay of molecular components, orchestrating a cascade of events that underlie immune responses and inflammation. Central to this pathway are several key players, including IL-17 receptors (IL-17RA and IL-17RC), as well as downstream signaling molecules such as Act1, TRAF6, and NF-κB.
IL-17 Receptors (IL-17RA and IL-17RC): The IL-17 receptor family forms the initial point of contact for IL-17 cytokines. Among them, IL-17RA and IL-17RC are pivotal in mediating IL-17 signaling. These receptors are predominantly expressed on various cell types, including epithelial cells, fibroblasts, and immune cells. Upon binding to IL-17, these receptors initiate a series of events that ultimately influence gene expression and cellular responses.
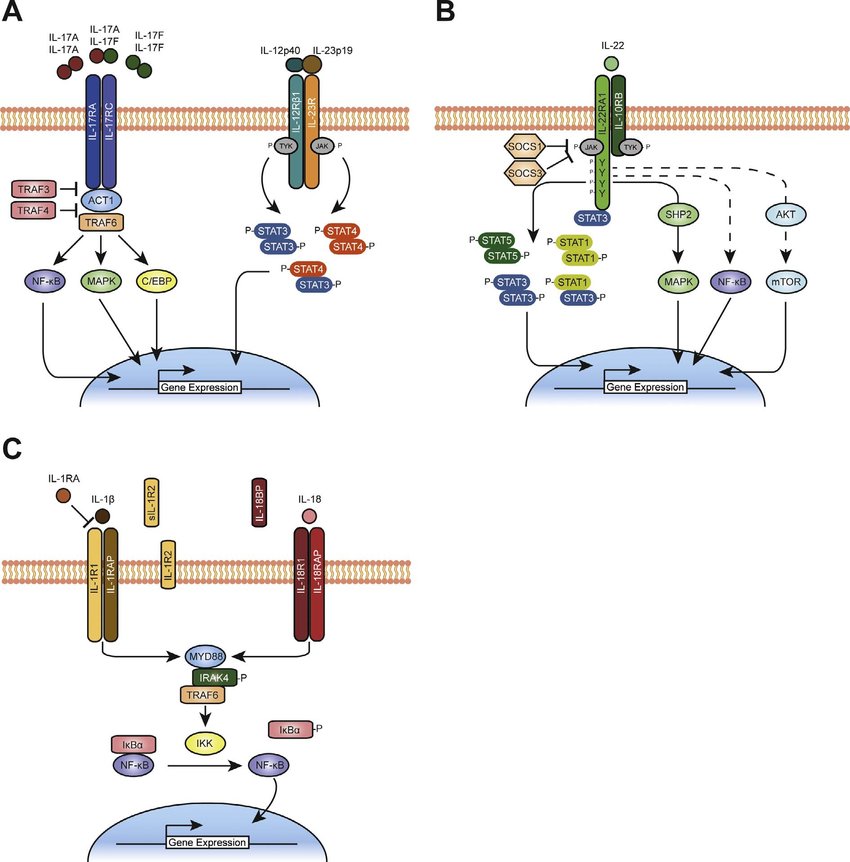 Signaling pathways of Th17-cell associated cytokines, and IL-1 and IL-18 (Nguyen et al., 2019).
Signaling pathways of Th17-cell associated cytokines, and IL-1 and IL-18 (Nguyen et al., 2019).
Act1 (Adapter for IL-17 Receptor Signaling): Act1 serves as a crucial adaptor molecule linking IL-17 receptors to downstream signaling pathways. After IL-17 engages its receptors, Act1 is recruited to the receptor complex. This recruitment triggers Act1's E3 ubiquitin ligase activity, leading to the formation of K63-linked polyubiquitin chains. These chains act as scaffolds for recruiting downstream signaling molecules.
TRAF6 (TNF Receptor-Associated Factor 6): TRAF6 plays a pivotal role in transducing IL-17 signals downstream. It interacts with the K63-linked polyubiquitin chains formed by Act1, leading to its activation. Activated TRAF6 then engages various signaling pathways, including the NF-κB and MAPK pathways. This activation results in the initiation of a transcriptional program that drives inflammatory responses.
NF-κB (Nuclear Factor-kappa B): The activation of NF-κB is a central event in IL-17 signaling. TRAF6-mediated activation of the IKK complex leads to the phosphorylation and subsequent degradation of IκBα, allowing NF-κB to translocate into the nucleus. In the nucleus, NF-κB binds to specific DNA sequences, promoting the transcription of pro-inflammatory genes responsible for the immune response and inflammation.
The interaction of these key components is a finely tuned process that culminates in the transduction of the IL-17 signal. Upon IL-17 binding to its receptors, a cascade of events is triggered: Act1 is recruited and activated, leading to the formation of K63-linked polyubiquitin chains. This, in turn, activates TRAF6, initiating downstream signaling through pathways like NF-κB. The activation of NF-κB results in the transcription of target genes involved in inflammation and immune regulation.
Select Service
Regulation of IL-17 Signaling
The IL-17 signaling pathway operates under stringent regulatory mechanisms to ensure the delicate balance of cytokine activity and prevent excessive immune responses. These regulatory processes involve a complex interplay of factors that modulate the pathway's activity, encompassing both positive and negative regulators, as well as intricate feedback loops.
Positive Regulation:
Several factors positively regulate IL-17 signaling to enhance immune responses when needed. One such factor is the interaction between IL-17 and IL-17 receptors. This interaction triggers the recruitment of adaptor molecules like Act1 and downstream signaling molecules, initiating a cascade of events that amplify the signal and promote pro-inflammatory gene expression.
Negative Regulation:
To maintain proper cytokine balance and prevent uncontrolled inflammation, the IL-17 signaling pathway is tightly regulated by various mechanisms. Cytokine inhibitors play a critical role in attenuating IL-17 signaling. For instance, IL-25 and IL-27 are known to suppress Th17 cell differentiation, thus indirectly dampening IL-17 production. Additionally, regulatory T cells (Tregs) produce anti-inflammatory cytokines like IL-10, which can inhibit Th17 responses and restrain IL-17 signaling.
Feedback Loops:
Feedback loops are integral to the fine-tuning of IL-17 signaling. One notable example involves IL-17-induced cytokines themselves. IL-17 stimulates the production of various cytokines, such as IL-6 and IL-23, which further promote Th17 differentiation and IL-17 secretion. This creates a positive feedback loop, intensifying the pathway's activity. However, prolonged activation of this loop can lead to excessive inflammation and immune dysregulation.
Inhibition by Other Signaling Pathways:
Cross-talk between signaling pathways also contributes to the regulation of IL-17 signaling. For instance, the anti-inflammatory cytokine IL-10, which is produced by immune cells like Tregs, suppresses IL-17 signaling by inhibiting the activation of downstream molecules like NF-κB. Similarly, pathways triggered by anti-inflammatory cytokines like transforming growth factor-beta (TGF-β) counterbalance IL-17-induced inflammation.
Epigenetic Regulation:
Epigenetic mechanisms also play a role in shaping IL-17 responses. DNA methylation and histone modifications can influence the expression of genes involved in the IL-17 pathway. These epigenetic changes can impact the differentiation and function of Th17 cells, thereby affecting IL-17 signaling.
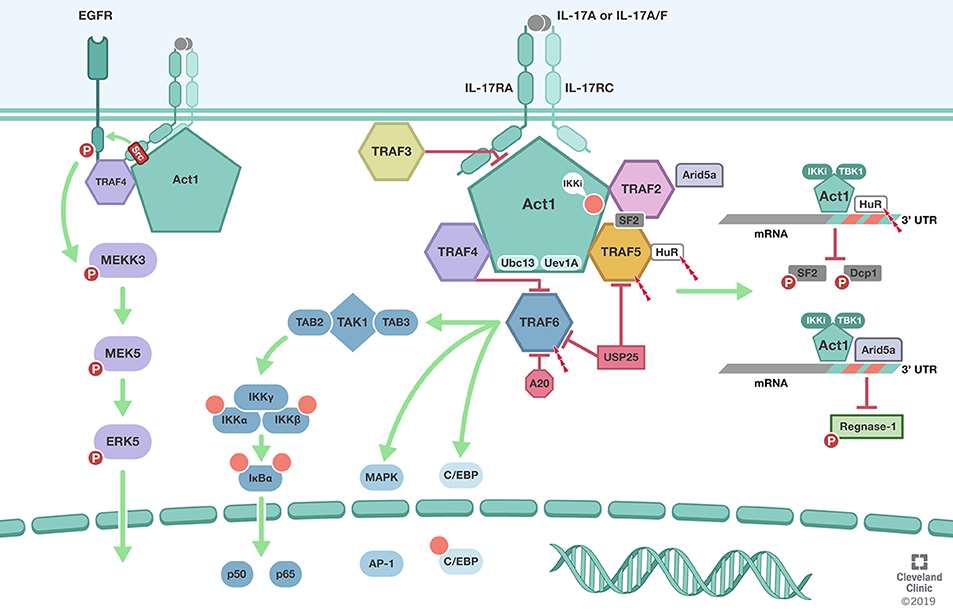 TRAF Regulation of
IL-17 Cytokine Signaling (Swaidani et al., 2019)
TRAF Regulation of
IL-17 Cytokine Signaling (Swaidani et al., 2019)
IL-17 Signaling in Various Diseases and Conditions
Autoimmune Diseases
IL-17's prominence in autoimmune diseases has spurred extensive research into its contribution to cytokine alterations. In psoriasis, IL-17 orchestrates an inflammatory milieu characterized by elevated levels of IL-17 itself, as well as pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6. This cytokine network fuels keratinocyte proliferation and the recruitment of immune cells, culminating in characteristic skin lesions.
Inflammatory Disorders
The impact of IL-17 on cytokine profiles is also evident in inflammatory disorders. Inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis, showcase elevated IL-17 levels alongside increased production of pro-inflammatory cytokines like IL-23 and IL-6. These cytokines contribute to gut inflammation and the perpetuation of IBD pathogenesis.
Infections and Immune Responses
IL-17's dynamic role in infections influences cytokine responses to invading pathogens. During fungal infections like candidiasis, IL-17 triggers cytokine alterations characterized by increased levels of IL-6 and IL-8, promoting neutrophil recruitment and anti-fungal defense. Conversely, bacterial infections can provoke aberrant cytokine responses, leading to tissue damage and immunopathology.
Cytokine Interplay
The multifaceted cytokine alterations triggered by IL-17 underscore its capacity to shape immune landscapes. The cytokine milieu in IL-17-related diseases often encompasses a symphony of pro-inflammatory cytokines, with IL-17 interacting synergistically with TNF-α, IL-6, and IL-1β to amplify inflammation. Conversely, anti-inflammatory cytokines like IL-10 aim to counterbalance IL-17-induced responses.
Emerging Biomarker Potential
The intricate cytokine alterations driven by IL-17 offer potential as biomarkers for disease characterization and prognosis. Monitoring changes in cytokine profiles, particularly those influenced by IL-17, can provide insights into disease progression and response to therapies. This holds promise for personalized medicine approaches tailored to individual cytokine profiles.
Techniques for Cytokine Analysis in the IL-17 Pathway
Luminex xMAP technology (Multi-Analyte Profiling) is a prominent cytokine analysis method within the IL-17 pathway. This innovative platform revolutionizes cytokine assessment by simultaneously measuring multiple cytokines from a single sample. Utilizing uniquely color-coded beads, each conjugated to a specific cytokine antibody, the xMAP system facilitates the capture and quantification of cytokines in a high-throughput manner.
Luminex xMAP technology offers exceptional advantages for studying the IL-17 pathway. Its multiplexing capability empowers researchers to analyze numerous cytokines concurrently, including those intricately associated with IL-17 responses. This approach provides a panoramic view of cytokine networks, unveiling the complex interplay and dynamics that govern immune responses.
Moreover, Luminex xMAP technology optimizes resource utilization, requiring minimal sample volume while providing a wealth of cytokine data. The platform's sensitivity and accuracy enhance our understanding of cytokine alterations within the IL-17 pathway, shedding light on their roles in disease and immunomodulation.
References:
- Nguyen, Paul M., and Tracy L. Putoczki. "Could the inhibition of IL-17 or IL-18 be a potential therapeutic opportunity for gastric cancer?" Cytokine 118 (2019): 8-18.
- Swaidani, Shadi, et al. "TRAF regulation of IL-17 cytokine signaling." Frontiers in immunology 10 (2019): 1293.