What are Equine Cytokines?
Cytokines are a diverse group of proteins, peptides, or glycoproteins secreted by specific cells of the immune system. These molecules act as messengers that facilitate communication between cells to trigger the body's response to infection, inflammation, and trauma. In horses, cytokines are critical in orchestrating the immune response, ensuring the effective elimination of pathogens, and maintaining immune balance.
Cytokines are central to the immune defense mechanisms in horses, influencing a wide range of physiological processes. Their importance extends beyond mere immune regulation to include roles in growth, development, and tissue repair. Unlike cytokines in other species, equine cytokines have unique aspects that reflect the specific evolutionary and physiological adaptations of horses. These differences underline the need for species-specific research and therapeutic strategies.
Basic Components of the Equine Immune System
The immune system of the equine serves as the primary defense mechanism against infectious agents, such as bacteria, viruses, fungi, and parasites. The system is composed of two main branches: the innate immune system and the adaptive immune system, each playing a distinct yet complementary role in maintaining the health and homeostasis of the horse.
Innate Immune System
- First Line of Defense: The innate immune system acts as the horse's initial barrier against pathogens. It is non-specific, meaning it responds to pathogens in a generalized manner without requiring prior exposure.
- Key Components: The innate immune system includes physical barriers like the skin and mucous membranes, as well as cellular defenses such as macrophages, neutrophils, and natural killer (NK) cells. These cells are equipped with pattern recognition receptors (PRRs) that detect common microbial signatures and initiate an immediate response.
- Inflammatory Response: Cytokines such as IL-1, IL-6, and TNF-α are rapidly produced upon activation of the innate immune system, leading to inflammation, which serves to isolate and destroy invading pathogens while signaling other immune cells to the site of infection.
Adaptive Immune System
- Tailored Defense: Unlike the innate immune system, the adaptive immune system is highly specific and capable of remembering past encounters with pathogens, which allows for a more efficient and potent response upon re-exposure. This immunological memory is a cornerstone of the adaptive immune response.
- Key Components: The adaptive immune system relies on lymphocytes, specifically T cells and B cells. T cells are further divided into helper T cells (Th cells), which assist other immune cells, and cytotoxic T cells, which directly kill infected cells. B cells are responsible for producing antibodies, which neutralize pathogens and mark them for destruction by other immune cells.
- Cytokine Involvement: Cytokines play a crucial role in regulating the adaptive immune response. For instance, IL-4 is essential for the differentiation of Th2 cells and the subsequent activation of B cells, while IL-12 is critical for promoting Th1 responses that are effective against intracellular pathogens.
Unique Features of Equine Immunity
The immune system of horses has evolved to address the unique challenges posed by their natural environment and lifestyle. These features distinguish the equine immune system from that of other mammals and are crucial for understanding how horses respond to infections and other immune challenges.
Evolutionary Adaptations
- Large Body Size and Longevity: Horses, being large and long-lived animals, have developed a robust immune system capable of dealing with chronic low-level exposure to pathogens. This evolutionary pressure has shaped a distinct cytokine response profile that emphasizes efficient pathogen clearance while minimizing tissue damage over the long term.
- Gastrointestinal Immunity: The equine gut is a critical site for immune activity, given the horse's dependence on a fiber-rich diet and the associated microbial load. The large intestine, in particular, harbors a complex community of microbes that contribute to both digestion and immune function. Cytokines such as IL-10 and TGF-β play significant roles in maintaining gut homeostasis and preventing excessive inflammation in response to the gut microbiota.
Response to Environmental Stressors
- Seasonal Variability: Horses often experience seasonal changes in immune function, influenced by factors such as temperature fluctuations, diet changes, and variations in pathogen exposure. For example, winter months may see an increase in respiratory infections, prompting a heightened cytokine response involving pro-inflammatory mediators like IL-1 and TNF-α.
- Exercise and Performance: Regular exercise and physical exertion, particularly in performance horses, can significantly impact immune function. Exercise-induced stress is known to alter cytokine profiles, potentially leading to a transient suppression of the immune system. Understanding these changes is critical for managing the health of sport horses and preventing opportunistic infections.
Classification and Types of Equine Cytokines
Pro-inflammatory Cytokines
Pro-inflammatory cytokines are primarily responsible for promoting inflammation, a critical aspect of the body's defense against infections and injuries. In horses, key pro-inflammatory cytokines include Interleukin-1 (IL-1), Interleukin-6 (IL-6), and Tumor Necrosis Factor-alpha (TNF-α).
- IL-1: Acts as a potent mediator of the inflammatory response, influencing the expression of other cytokines, chemokines, and adhesion molecules.
- IL-6: Plays a dual role by contributing to inflammation and stimulating immune responses, particularly the acute phase response.
- TNF-α: A major cytokine involved in systemic inflammation, responsible for inducing fever, apoptosis, and other aspects of the immune response.
These cytokines are crucial in the pathogenesis of several equine diseases, including septicemia, colic, and laminitis, where excessive inflammation can lead to severe tissue damage.
Anti-inflammatory Cytokines
Anti-inflammatory cytokines serve as a counterbalance to pro-inflammatory cytokines, helping to control and resolve inflammation. Key anti-inflammatory cytokines in horses include Interleukin-10 (IL-10) and Transforming Growth Factor-beta (TGF-β).
- IL-10: Suppresses the expression of pro-inflammatory cytokines, inhibits antigen presentation, and regulates immune responses to prevent tissue damage.
- TGF-β: Plays a multifaceted role by promoting wound healing, regulating immune tolerance, and inhibiting the proliferation of T cells.
The balance between pro-inflammatory and anti-inflammatory cytokines is critical in maintaining immune homeostasis and preventing chronic inflammation, which can lead to conditions such as equine arthritis and chronic obstructive pulmonary disease (COPD).
Regulatory and Modulatory Cytokines
Regulatory cytokines are involved in fine-tuning the immune response, ensuring that it is appropriate in magnitude and duration. In horses, Interleukin-2 (IL-2), Interleukin-12 (IL-12), and Interferon-gamma (IFN-γ) are prominent examples.
- IL-2: Essential for T cell proliferation, differentiation, and survival, playing a central role in the adaptive immune response.
- IL-12: Promotes the differentiation of naive T cells into Th1 cells, which are crucial for the defense against intracellular pathogens.
- IFN-γ: Produced primarily by T cells and natural killer (NK) cells, IFN-γ activates macrophages, enhances antigen presentation, and modulates the adaptive immune response.
These cytokines are vital in modulating the immune response, particularly in ensuring that the response is efficient but does not become excessive, which could lead to autoimmune reactions.
Chemokines and Growth Factors
Chemokines and growth factors are specialized cytokines that guide the movement of immune cells to sites of infection or injury and promote tissue repair. Notable examples in horses include CCL2 (Monocyte Chemoattractant Protein-1) and CXCL8 (Interleukin-8).
- CCL2: Attracts monocytes to sites of inflammation, where they differentiate into macrophages and participate in the immune response.
- CXCL8: Functions primarily to attract neutrophils, which are the first responders during an infection.
Growth factors, such as Platelet-Derived Growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF), are critical in tissue regeneration and wound healing. They promote the proliferation and migration of cells necessary for repairing damaged tissues, thus playing a crucial role in equine wound management.
Services you may be interested in:
Cytokine Production, Signaling, and Mechanisms in Horses
Cellular Sources of Cytokines
In equine biology, cytokine production is attributed to a diverse array of cells, encompassing macrophages, T cells, B cells, endothelial cells, and fibroblasts. The synthesis of cytokines is typically initiated upon the recognition of pathogens by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs). Upon activation, these cells secrete cytokines that function in autocrine, paracrine, or endocrine capacities to coordinate the immune response.
- Macrophages: Represent a principal source of pro-inflammatory cytokines during the innate immune response.
- T Cells: Generate a broad spectrum of cytokines, pivotal in modulating the adaptive immune response, especially during pathogen-specific immune reactions.
- Endothelial Cells and Fibroblasts: Contribute to the production of cytokines and growth factors that are integral to inflammation, wound healing, and tissue remodeling processes.
Cytokine Receptors and Signaling Pathways
Cytokines exert their biological effects by binding to specific receptors located on the surface of target cells. This interaction initiates a cascade of intracellular signaling events, leading to the activation of transcription factors and culminating in the expression of genes that drive the immune response.
- JAK-STAT Pathway: A central signaling mechanism activated by cytokine receptors. The binding of cytokines such as IFN-γ to their respective receptors activates Janus kinases (JAKs), which subsequently phosphorylate Signal Transducer and Activator of Transcription (STAT) proteins. These phosphorylated STATs then translocate to the nucleus to modulate gene expression.
- MAPK Pathway: Engaged by cytokines such as TNF-α, this pathway is involved in regulating cell proliferation, differentiation, and apoptosis.
- NF-κB Pathway: A crucial pathway in immune response regulation, inflammation, and cell survival. Pro-inflammatory cytokines like IL-1 and TNF-α activate NF-κB, leading to the transcription of genes implicated in the inflammatory response.
The precise regulation of these signaling pathways is essential, as dysregulation can result in aberrant immune responses, contributing to the pathogenesis of various diseases.
Regulation of Cytokine Expression
Cytokine expression in horses is tightly regulated by a confluence of genetic, epigenetic, and environmental factors.
- Genetic Regulation: The expression of cytokines and their receptors is governed by specific genes, and genetic polymorphisms can influence cytokine levels and activity. For instance, polymorphisms in the TNF-α gene have been linked to variations in disease susceptibility and severity in horses.
- Epigenetic Regulation: Epigenetic mechanisms, such as DNA methylation and histone acetylation, can modulate cytokine gene expression without altering the underlying DNA sequence. These epigenetic changes are influenced by environmental factors, including diet, stress, and infection.
- Environmental Influences: External stimuli such as infection, injury, and stress can significantly impact cytokine production. For example, during an infection, microbial products like lipopolysaccharides (LPS) can induce a strong cytokine response through the activation of TLRs.
The regulation of cytokine expression is a dynamic and tightly controlled process that can contribute to the initiation of an effective immune response and ensure its resolution, thereby preventing chronic inflammation and autoimmune conditions.
Role of Cytokines in Equine Disease
Cytokines in Infectious Diseases
Cytokines are at the forefront of the immune response to infectious agents in horses. Their levels and activity are critical in determining the outcome of infections, influencing whether an immune response will successfully eliminate the pathogen or lead to chronic disease.
- Strangles (Streptococcus equi infection): Characterized by an overproduction of pro-inflammatory cytokines such as IL-1 and TNF-α, which contribute to severe inflammation and abscess formation.
- Equine Influenza: Involves a complex cytokine response, including both pro-inflammatory cytokines like IL-6 and anti-inflammatory cytokines to modulate the immune response and minimize tissue damage.
- West Nile Virus: A viral infection that triggers a strong immune response, including the production of interferons (e.g., IFN-α and IFN-β) to inhibit viral replication and activate antiviral defenses.
Understanding cytokine responses in these infections helps in developing targeted therapies and vaccines to improve disease management and prevention.
Cytokines in Inflammatory and Autoimmune Disorders
Inappropriate or excessive cytokine responses can lead to chronic inflammation and autoimmune disorders in horses.
- Equine Arthritis: Chronic inflammation in arthritis is often driven by pro-inflammatory cytokines such as IL-1 and TNF-α, which perpetuate joint inflammation and cartilage degradation.
- Equine Recurrent Airway Obstruction (RAO): Characterized by an exaggerated inflammatory response to environmental allergens, with elevated levels of IL-4 and IL-13 contributing to airway inflammation and hyperreactivity.
Targeting specific cytokines involved in these conditions can help alleviate symptoms and improve quality of life for affected horses.
Cytokines in Allergies and Hypersensitivity
Allergic reactions in horses are mediated by cytokines that regulate the immune response to allergens.
- Equine Asthma: Involves an imbalance of cytokines such as IL-4 and IL-13, which drive the Th2-mediated allergic response and contribute to bronchial inflammation and mucus production.
Interventions that modulate these cytokines can provide relief from allergic symptoms and improve respiratory health in affected horses.
Cytokines in Trauma and Wound Healing
Cytokines play a crucial role in the healing process following injury, influencing inflammation, tissue repair, and fibrosis.
- Wound Healing: Cytokines such as TGF-β and PDGF promote cell proliferation, migration, and extracellular matrix production, facilitating effective tissue repair. However, excessive cytokine activity can lead to abnormal healing or fibrosis.
Understanding the role of cytokines in wound healing allows for the development of treatments that enhance repair processes and minimize scarring.
Therapeutic Targeting of Cytokines
Cytokine-targeted therapies offer potential benefits in treating various equine diseases by modulating aberrant cytokine activity.
- Cytokine Inhibitors: Agents that block specific cytokines or their receptors (e.g., anti-TNF-α antibodies) are used to manage chronic inflammatory conditions like arthritis.
- Gene Therapy: Emerging approaches aim to correct cytokine imbalances at the genetic level, offering potential long-term solutions for autoimmune and inflammatory disorders.
Equine Cytokine Analysis Methods
Accurate analysis of cytokines in the horse helps us understand the immune response and disease mechanisms. Various techniques are used for cytokine studies. We can make suitable choices based on their respective characteristics.
Enzyme-Linked Immunosorbent Assay (ELISA)
Enzyme-Linked Immunosorbent Assay (ELISA) is one of the most commonly used techniques for cytokine quantification in equine studies. ELISA works by detecting cytokines through their specific interaction with antibodies. After the target cytokine binds to an antibody, a secondary antibody, often linked to an enzyme, is introduced. Upon adding a substrate, the enzyme catalyzes a color change, which is measurable and directly proportional to the cytokine concentration.
ELISA is highly valued for its specificity and sensitivity, allowing for the detection of cytokines even at very low concentrations. It is particularly useful in both research and clinical settings for monitoring cytokine levels in serum, plasma, or other biological fluids. However, a significant limitation of ELISA is its typically single-target focus, meaning that only one cytokine can be measured per assay. This constraint makes it less efficient when dealing with the complex cytokine networks often involved in immune responses.
Flow Cytometry
Flow cytometry, especially when combined with intracellular cytokine staining, offers a powerful approach to cytokine analysis at the single-cell level. This technique involves labeling cells with fluorescent antibodies that bind to specific cytokines. The cells are then passed through a laser beam, and the emitted fluorescence is measured to determine the presence and quantity of cytokines within individual cells.
Flow cytometry excels in providing detailed information about the cellular sources of cytokines and allows for the simultaneous measurement of multiple cytokines within a single cell population. This capability is crucial for understanding the dynamics of cytokine production in different immune cell subsets. However, flow cytometry requires sophisticated equipment and expertise, and the process can be complex, making it less accessible for routine analysis in some settings.
Luminex xMAP Technology
Luminex xMAP technology (Multi-Analyte Profiling) has revolutionized cytokine analysis by enabling the simultaneous quantification of multiple cytokines from a single sample. This bead-based multiplexing system uses microspheres that are internally dyed with different ratios of fluorescent colors, each coupled with an antibody specific to a target cytokine. When the beads are incubated with a biological sample, cytokines bind to their corresponding beads. A secondary antibody tagged with a fluorescent dye is then added, producing a signal proportional to the cytokine concentration. The signals are detected using a flow-based analyzer, which reads the fluorescence intensity and identifies the cytokine bound to each bead.
The primary advantage of Luminex xMAP technology is its ability to perform high-throughput, multiplex cytokine analysis, allowing for the measurement of up to 100 different cytokines in a single assay. This efficiency makes it an invaluable tool for comprehensive cytokine profiling in equine research, where understanding the complex interplay of cytokines is critical. Luminex xMAP is also highly sensitive and specific, with minimal cross-reactivity, enabling accurate quantification even in complex biological samples like serum or plasma. Additionally, it requires only a small volume of sample, which is particularly advantageous when sample quantity is limited. The speed and cost-effectiveness of Luminex xMAP further contribute to its popularity in both research and clinical diagnostics.
Services you may be interested in:
Quantitative Polymerase Chain Reaction (qPCR)
Quantitative Polymerase Chain Reaction (qPCR) is another key technique used in cytokine research, though it focuses on the measurement of cytokine mRNA levels rather than proteins. qPCR works by amplifying specific cytokine genes from a sample, allowing researchers to quantify the amount of mRNA present. This method provides insights into the transcriptional regulation of cytokines and can indicate how gene expression changes in response to different conditions, such as infection or inflammation.
The primary advantage of qPCR lies in its sensitivity and ability to detect changes in cytokine gene expression with high precision. It is especially useful for studying cytokine regulation at the genetic level, offering a glimpse into the upstream mechanisms controlling cytokine production. However, one limitation is that mRNA levels do not always correlate with protein levels due to post-transcriptional modifications and other regulatory processes. Therefore, while qPCR provides valuable information about gene expression, it may not fully reflect the actual cytokine activity in the body.
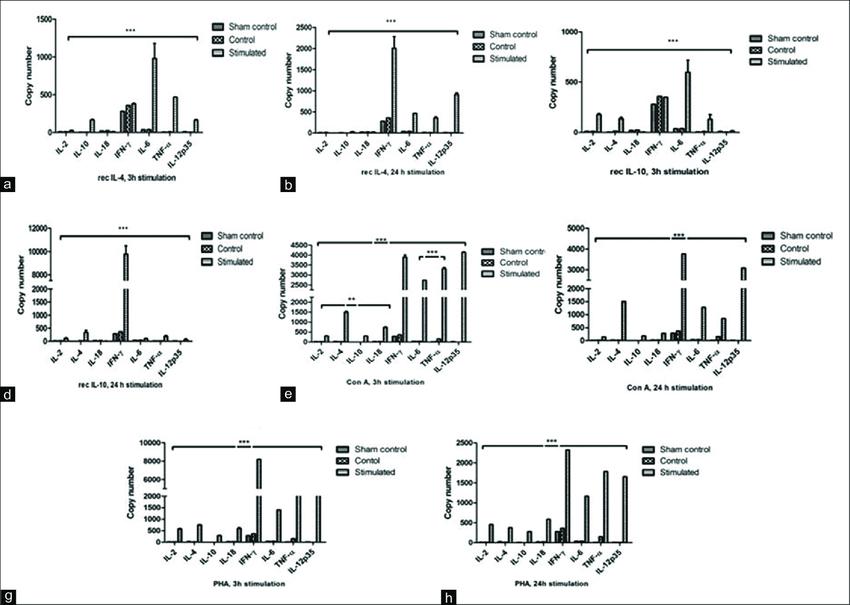 Quantification of equine cytokines in horse peripheral blood mononuclear cells (Saini et al, 2019).
Quantification of equine cytokines in horse peripheral blood mononuclear cells (Saini et al, 2019).
Western Blotting
Western blotting is a widely used technique for the detection and quantification of cytokine proteins. This method separates proteins based on their molecular weight through gel electrophoresis, after which the proteins are transferred onto a membrane. The membrane is then probed with specific antibodies against the cytokine of interest. Detection is achieved through the use of secondary antibodies conjugated to enzymes or fluorescent markers, allowing for visualization and quantification of the target protein.
Western blotting is particularly useful for confirming the presence of specific cytokines and assessing their relative abundance. It also provides valuable information on post-translational modifications, such as phosphorylation, which can influence cytokine activity. However, the technique is relatively labor-intensive and less suited for high-throughput analysis, making it more appropriate for targeted studies rather than broad cytokine profiling.
Reference:
- Saini, Sheetal, et al. "Recombinant horse interleukin-4 and interleukin-10 induced a mixed inflammatory cytokine response in horse peripheral blood mononuclear cells." Veterinary World 12.4 (2019): 496.

