Background of Neurological Diseases
Neurological diseases encompass a broad range of disorders that affect the central nervous system (CNS) and peripheral nervous system (PNS), leading to dysfunctions in cognition, movement, sensation, and autonomic processes. These diseases can be classified into several major categories, including neurodegenerative diseases, autoimmune disorders, cerebrovascular diseases, neuroinfectious diseases, and neurodevelopmental disorders.
Neurological diseases are among the leading causes of disability and mortality worldwide. According to the World Health Organization (WHO), neurological disorders account for a significant portion of the global disease burden, with conditions such as Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), stroke, and epilepsy affecting millions of people. The prevalence of neurodegenerative diseases, particularly AD and PD, is increasing due to aging populations, making them a major public health concern.
Classification of Neurological Diseases
Neurodegenerative Diseases
Neurodegenerative diseases are characterized by the progressive loss of neurons, leading to cognitive and motor impairments. These diseases are often associated with protein misfolding, oxidative stress, mitochondrial dysfunction, and neuroinflammation. Major neurodegenerative disorders include:
- Alzheimer's Disease (AD): Characterized by amyloid-beta (Aβ) plaque accumulation and tau protein hyperphosphorylation, leading to memory loss and cognitive decline.
- Parkinson's Disease (PD): Involves the degeneration of dopaminergic neurons in the substantia nigra, resulting in motor dysfunction such as tremors, rigidity, and bradykinesia.
- Amyotrophic Lateral Sclerosis (ALS): Affects motor neurons in the brain and spinal cord, leading to muscle weakness and paralysis.
- Huntington's Disease (HD): A genetic disorder caused by CAG trinucleotide repeat expansions, leading to movement disorders and psychiatric symptoms.
Autoimmune Neurological Disorders
These diseases occur when the immune system mistakenly attacks components of the nervous system, causing inflammation and demyelination. Key disorders include:
- Multiple Sclerosis (MS): An autoimmune condition in which the immune system attacks the myelin sheath of neurons, leading to demyelination and neurological dysfunction.
- Neuromyelitis Optica Spectrum Disorder (NMOSD): A severe inflammatory disorder primarily affecting the optic nerves and spinal cord.
- Myasthenia Gravis (MG): Caused by autoantibodies against acetylcholine receptors, leading to muscle weakness.
Cerebrovascular Diseases
These diseases result from impaired blood flow to the brain, leading to neuronal damage or death. Common conditions include:
- Stroke: Caused by a blockage (ischemic stroke) or rupture (hemorrhagic stroke) of blood vessels in the brain, leading to neurological deficits.
- Vascular Dementia: Cognitive decline caused by chronic ischemic damage to the brain.
Neuroinfectious Diseases
These are caused by viruses, bacteria, fungi, or parasites that invade the nervous system. Examples include:
- Meningitis: Inflammation of the meninges, typically caused by bacterial or viral infections.
- Encephalitis: Inflammation of the brain, often caused by viral infections such as herpes simplex virus (HSV).
- HIV-Associated Neurocognitive Disorders (HAND): A spectrum of neurological impairments in HIV-infected individuals.
Neurodevelopmental Disorders
These disorders arise from abnormalities in brain development and are often diagnosed in childhood. Examples include:
- Autism Spectrum Disorder (ASD): A developmental disorder characterized by impaired social communication and repetitive behaviors.
- Attention-Deficit/Hyperactivity Disorder (ADHD): Marked by inattention, hyperactivity, and impulsivity.
- Cerebral Palsy (CP): A group of movement disorders caused by brain damage during fetal development or infancy.
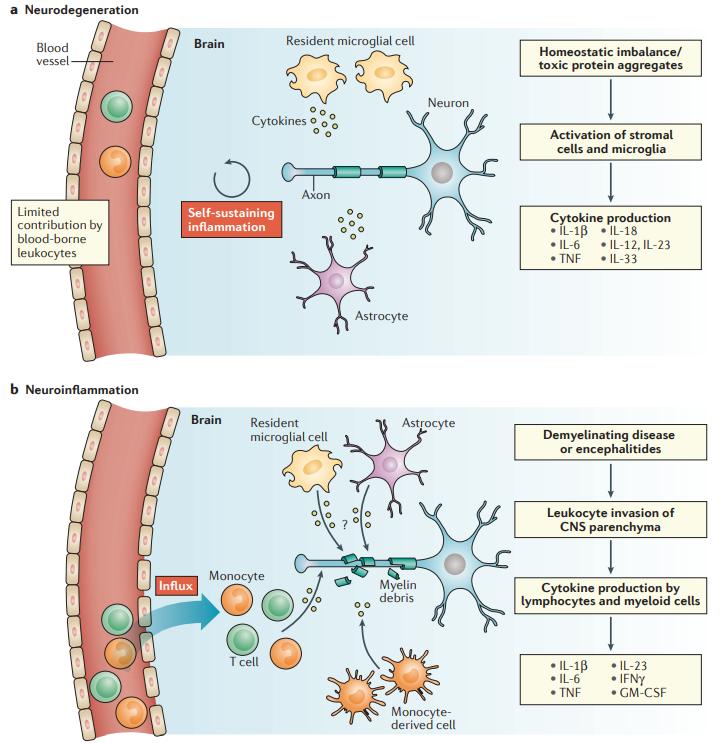 Neurodegeneration versus neuroinflammation (Becher et al., 2017).
Neurodegeneration versus neuroinflammation (Becher et al., 2017).
Mechanism of Cytokines in Neurological Diseases
Cytokines are key mediators in the immune system and play a crucial role in the regulation of neuroinflammatory responses. In neurological diseases, cytokines are involved in neuroinflammation, neuronal survival and death, synaptic function, and blood-brain barrier integrity. The complex interplay between pro-inflammatory and anti-inflammatory cytokines determines the extent of neuronal damage or protection.
Neuroinflammation and Microglial Activation
Neuroinflammation is a hallmark of many neurological diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). In these conditions, immune cells—especially microglia and astrocytes—become activated and release a cascade of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6).
- Microglial activation: Microglia are the resident immune cells of the central nervous system (CNS). Under pathological conditions, microglia transition from a homeostatic state to a reactive state, releasing pro-inflammatory cytokines that amplify neuroinflammation. Chronic microglial activation leads to sustained production of TNF-α, IL-1β, and interferon-gamma (IFN-γ), which can promote neuronal dysfunction and death.
- Astrocyte involvement: Astrocytes, another type of glial cell, can be activated by cytokines such as IL-1β and TNF-α. Reactive astrocytes contribute to the formation of a neurotoxic environment by secreting glutamate, reactive oxygen species (ROS), and nitric oxide (NO), which further exacerbate neuronal damage.
Cytokine-Mediated Neuronal Damage and Apoptosis
Excessive levels of pro-inflammatory cytokines can induce neurodegeneration through various mechanisms:
- Direct toxicity to neurons: TNF-α and IL-1β activate apoptotic pathways through death receptors (such as TNF receptor 1, TNFR1), leading to caspase activation and neuronal apoptosis.
- Mitochondrial dysfunction: Cytokines such as TNF-α and IL-6 contribute to mitochondrial dysfunction by increasing oxidative stress and impairing energy metabolism, which makes neurons more susceptible to apoptosis.
- Excitotoxicity: Pro-inflammatory cytokines can induce excessive glutamate release by reducing the expression of glutamate transporters on astrocytes, leading to excitotoxicity, which damages neurons through prolonged activation of NMDA receptors and calcium overload.
Disruption of the Blood-Brain Barrier (BBB)
The blood-brain barrier (BBB) is crucial for maintaining CNS homeostasis by preventing the infiltration of peripheral immune cells and harmful molecules. However, in many neurological diseases, cytokines play a role in BBB disruption:
- Increased permeability: Pro-inflammatory cytokines, such as TNF-α and IL-6, can downregulate tight junction proteins (e.g., occludin and claudin-5), making the BBB more permeable.
- Infiltration of peripheral immune cells: Increased BBB permeability allows peripheral immune cells, including T cells and monocytes, to enter the CNS, further amplifying neuroinflammation. This is particularly evident in multiple sclerosis (MS), where autoreactive T cells and macrophages infiltrate the CNS and attack myelin sheaths.
- Endothelial dysfunction: IL-1β and TNF-α can induce endothelial cell apoptosis and reduce vascular integrity, contributing to neurovascular damage.
Cytokine Effects on Synaptic Function and Plasticity
Cytokines also influence synaptic function, which is critical for learning, memory, and cognition. The dysregulation of cytokines in neurological diseases can impair synaptic plasticity in several ways:
- Inhibition of long-term potentiation (LTP): Pro-inflammatory cytokines, such as IL-1β and TNF-α, have been shown to impair synaptic plasticity by reducing LTP, which is essential for memory formation.
- Alterations in neurotransmitter balance: IL-6 and TNF-α can affect neurotransmitter systems by reducing dopamine levels in PD and modulating glutamate and GABA activity in AD and MS.
- Synaptic pruning: While microglia-mediated synaptic pruning is necessary for normal brain development, excessive cytokine signaling (such as IL-1β and C1q activation) in diseases like Alzheimer's disease can lead to the loss of functional synapses.
Dual Role of Anti-Inflammatory Cytokines
While pro-inflammatory cytokines contribute to neuronal damage, certain anti-inflammatory cytokines help counteract these effects:
- Interleukin-10 (IL-10): IL-10 suppresses the production of pro-inflammatory cytokines and inhibits microglial activation. However, in many neurological diseases, IL-10 levels are insufficient to control chronic inflammation.
- Transforming growth factor-beta (TGF-β): TGF-β promotes neuroprotection by inhibiting immune activation and supporting tissue repair. However, its role is complex, as excessive TGF-β signaling can also contribute to fibrosis and gliosis in neurodegenerative diseases.
- IL-4 and IL-13: These cytokines promote an M2 microglial phenotype, which is associated with tissue repair and anti-inflammatory effects. However, in chronic diseases, the balance between M1 (pro-inflammatory) and M2 (anti-inflammatory) microglia is often disrupted, leading to persistent inflammation.
Cytokine-Mediated Neurodegenerative Disease Progression
Cytokines play a central role in the progression of major neurological disorders:
- Alzheimer's disease (AD): Chronic neuroinflammation in AD is driven by microglial activation and elevated levels of IL-1β, TNF-α, and IL-6. These cytokines contribute to amyloid-beta (Aβ) plaque formation and tau pathology, accelerating neurodegeneration.
- Parkinson's disease (PD): Increased levels of IL-1β, TNF-α, and IFN-γ contribute to the degeneration of dopaminergic neurons in the substantia nigra, exacerbating motor symptoms.
- Multiple sclerosis (MS): In MS, pro-inflammatory cytokines such as IL-17 and IFN-γ drive autoimmunity, leading to demyelination and neurodegeneration.
- Amyotrophic lateral sclerosis (ALS): Elevated IL-6 and TNF-α contribute to motor neuron death, leading to muscle weakness and paralysis.
Analyzing Cytokines in Neurological Diseases
Sample Collection and Preparation
Sample Types
In neurological research, samples can include cerebrospinal fluid (CSF), serum, or even brain tissue homogenates. The choice depends on the disease under study and the specific cytokines of interest.
Pre-analytical Considerations
- Stability: Cytokines are sensitive to degradation; hence, rapid processing and proper storage (e.g., at –80°C) are essential.
- Minimizing Artifacts: Using protease inhibitors and ensuring minimal freeze-thaw cycles can help maintain sample integrity.
Selection of Cytokine Panels
| Species | Specification | Protein Targets | Price |
|---|---|---|---|
| Mouse | Mouse Neurodegenerative Disease 7-plex Panel | C-Reactive Protein/CRP, CCL5/RANTES, PDGF-AA, PDGF-BB, RAGE/AGER, Serpin E1/PAI-1, ICAM-1/CD54 | +Inquiry |
| Human | Human Neurodegenerative Disorders Biomarker 5-plex Panel | Aβ1-40, Aβ1-42, GDNF, S100B, sRAGE | +Inquiry |
| Human | Human Neurodegenerative Disorders Biomarker 6-plex Panel | α-2-Antitrypsin, Complement C4, CRP, MIP-4/PARC, Serum Amyloid P (SAP), PEDF | +Inquiry |
| Human | Human Neurodegenerative Disorders Biomarker 7-plex Panel | α-2-Macroglobulin, ApoA I, ApoC III, ApoE, Complement C3, Complement factor H (CFH), Prealbumin/Transthyretin (TTR) | +Inquiry |
| Human | Human Neurodegenerative Disorders Biomarker 10-plex Panel | BDNF, CathepsinD, Myeloperoxidase (MPO), PAI-1 (total), PDGF-AA, PDGF-AB/BB, RANTES/CCL5, sICAM-1, sNCAM, sVCAM-1 | +Inquiry |
| Human | Human Neurological Disorders Biomarker 9-plex Panel | Ceruloplasmin (CP), Haptoglobin (HP), Serum Amyloid P, α-1-AcidGlycoprotein (AGP) | +Inquiry |
| Human | Human Neurological Disorders Biomarker 11-plex Panel | Angiotensinogen (AGT), Contactin-1, FetuinA, Kallikrein-6, Osteopontin (OPN), Soluble Superoxide Dismutase1 (sSOD1), Soluble Superoxide Dismutase2 (sSOD2) | +Inquiry |
| Human | Human Neuropeptide 6-plex Panel | Neurotensin, OrexinA, Oxytocin, SubstanceP, α-MSH, β-Endorphin | +Inquiry |
We can also provide other customized panels, contact us to learn more!
Multiplex Assay Technologies
The Luminex platform is well-suited for cytokine profiling due to its ability to simultaneously quantify multiple analytes from a single small-volume sample. This technology utilizes bead-based multiplexing combined with fluorescent readouts, offering high sensitivity and dynamic range.
Data Analysis and Interpretation
Normalization and Quality Control
Raw data from multiplex assays need to be normalized to account for variability across samples. Implementing internal controls and standards is essential for accurate quantification.
Statistical Analysis
Use appropriate statistical tools to compare cytokine levels between diseased and control groups. Multivariate analyses can help reveal complex cytokine interaction patterns that may contribute to disease pathology.
Integration with Clinical Data
Combining cytokine profiles with clinical parameters (e.g., disease stage, cognitive scores) and other biomarkers can provide insights into the pathophysiological mechanisms driving neurological disorders.
Services you may be interested in:
Reference:
- Becher, Burkhard, Sabine Spath, and Joan Goverman. "Cytokine networks in neuroinflammation." Nature Reviews Immunology 17.1 (2017): 49-59.

