The immune system, a marvel of biological defense, orchestrates a complex network of cells and molecules to protect the body against a myriad of threats. At the heart of this intricate defense mechanism lie signaling molecules known as cytokines. These molecular messengers play a pivotal role in regulating immune responses, ensuring a delicate balance between defense against pathogens and tolerance to self.
Cytokines, in essence, are small proteins produced by various cells in the immune system. They serve as signaling molecules, facilitating communication between immune cells to coordinate and modulate their activities. The immune system, on the other hand, comprises a vast array of cells, tissues, and organs working collaboratively to identify and eliminate foreign invaders while distinguishing self from non-self.
The intricate interplay between cytokines and the immune system forms the crux of research in infectious diseases, autoimmune disorders, and transplantation medicine. Understanding this dynamic relationship is essential for unraveling the mysteries behind diseases caused by infectious agents, the aberrations in immune responses leading to autoimmune conditions, and the challenges encountered in organ and tissue transplantation.
Infectious diseases, characterized by invading pathogens such as viruses and bacteria, necessitate a detailed exploration of how cytokines regulate the immune response. Unraveling the intricacies of cytokine involvement provides insights into potential therapeutic interventions and vaccine development, crucial for combating infectious agents effectively.
In the realm of autoimmune diseases, where the immune system mistakenly targets and attacks the body's own tissues, cytokines emerge as key players in orchestrating these misguided responses. Investigating the role of cytokines in autoimmune conditions not only deepens our understanding of disease mechanisms but also opens avenues for targeted therapies to restore immune balance.
In the field of transplantation, where the immune system faces the challenge of distinguishing between self and foreign tissues, cytokine research becomes imperative. Understanding how cytokines influence the delicate balance between acceptance and rejection of transplanted organs holds the promise of refining immunosuppressive strategies and improving transplant outcomes.
Cytokine Basic Functions in Immunoregulation
Immunoregulatory Signaling: At its core, the primary function of cytokines lies in their role as signaling molecules that transmit information among immune cells. They act as messengers, conveying critical instructions to regulate the activation, proliferation, and differentiation of immune cells, ensuring a coordinated and effective defense against pathogens.
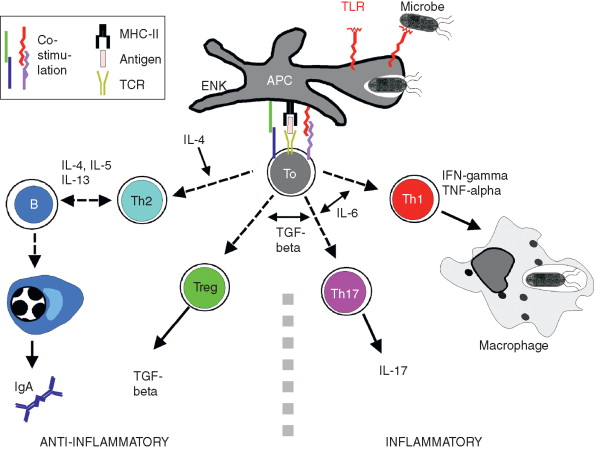 Generation of T- and B-effector cells in CALT (Knop et al., 2011).
Generation of T- and B-effector cells in CALT (Knop et al., 2011).
Modulation of Inflammatory Responses: Cytokines play a pivotal role in modulating inflammatory processes, serving as both pro-inflammatory and anti-inflammatory agents. Pro-inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α), initiate and amplify immune responses to eliminate pathogens. Conversely, anti-inflammatory cytokines, like interleukin-10 (IL-10), help resolve inflammation and prevent excessive tissue damage.
Chemotaxis and Cell Migration: A critical facet of the immune response involves the recruitment of immune cells to sites of infection or tissue damage. Cytokines known as chemokines, a subset of these signaling molecules, act as chemoattractants, guiding immune cells to specific locations. This chemotactic function is essential for the effective deployment of immune cells to areas where their presence is required.
Cell Growth and Differentiation: Cytokines exert control over the growth and differentiation of immune cells, ensuring a diverse and adaptable immune repertoire. Growth factors, a subset of cytokines, stimulate cell proliferation and influence the development of immune cells, contributing to the generation of specialized cell types with distinct functions.
Regulation of Apoptosis: Maintaining the delicate balance between cell survival and programmed cell death (apoptosis) is vital for proper immune function. Cytokines play a role in regulating apoptosis, influencing the life span of immune cells and facilitating the removal of damaged or unnecessary cells to uphold immune system integrity.
Coordination of Adaptive and Innate Immunity: Cytokines serve as mediators between the innate and adaptive arms of the immune system, facilitating communication between different immune cell types. This coordination is essential for mounting effective and targeted immune responses tailored to specific pathogens or challenges.
Select Service
Cytokines and Infectious Diseases
Cellular cytokines play a crucial role in the immune response against infectious diseases, including both viral and bacterial infections. These small proteins are essential for regulating various immune processes and mediating communication between different cells of the immune system.
In the context of viral infections, cytokines are key players in orchestrating the body's defense mechanisms. Upon viral invasion, infected cells release signaling molecules such as interferons to alert neighboring cells of the threat. Interferons induce an antiviral state in surrounding cells, inhibiting viral replication and spread. Additionally, other cytokines, such as interleukins and tumor necrosis factor (TNF), mobilize immune cells like T cells and macrophages to the site of infection, enhancing the immune response.
Similarly, during bacterial infections, cytokines play a vital role in modulating the immune system's activity. Pathogen-associated molecular patterns (PAMPs) from bacteria can trigger the release of cytokines such as interleukin-1 (IL-1) and TNF. These cytokines contribute to the recruitment of immune cells and the activation of the inflammatory response, essential for eliminating the bacterial threat. Furthermore, some cytokines are involved in the maturation and activation of B cells, which produce antibodies to neutralize bacteria.
Research on cytokines is of paramount importance in the quest for new therapeutic approaches and vaccine development. Understanding how cytokines regulate immune responses allows scientists to design targeted interventions that can enhance the body's ability to combat infections. In the case of viral infections, manipulating cytokine responses may help in controlling viral replication and limiting the severity of the disease. For bacterial infections, modulating cytokine production can aid in optimizing the immune response, potentially reducing the risk of complications.
In the realm of vaccine development, knowledge of cytokine dynamics is crucial for designing vaccines that elicit robust and protective immune responses. Vaccines often aim to stimulate the production of specific cytokines that enhance the activation of immune cells and the generation of long-lasting immunity.
Select Service
Role of Cytokines in Autoimmune Diseases
Cytokines play a crucial role in the regulation of the immune system and are closely associated with the development and progression of autoimmune diseases. Autoimmune diseases occur when the immune system mistakenly recognizes the body's own tissues as foreign and mounts an immune response against them. The involvement of cytokines in this process is multifaceted, contributing to both the initiation and perpetuation of autoimmune responses.
Initiation of Autoimmune Response
- Th1 and Th17 Responses: Certain cytokines, such as interferon-gamma (IFN-γ) and interleukin-17 (IL-17), promote the differentiation and activation of T helper 1 (Th1) and T helper 17 (Th17) cells. These cells play a key role in initiating immune responses against self-antigens.
- Antigen-Presenting Cell Activation: Cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1), activate antigen-presenting cells (APCs) such as macrophages and dendritic cells. Activated APCs present self-antigens to T cells, leading to the initiation of an autoimmune response.
Amplification of Immune Response
- Cytokine Feedback Loops: In autoimmune diseases, there is often a dysregulation of cytokine production, leading to the formation of positive feedback loops. This results in sustained activation and proliferation of immune cells, exacerbating the autoimmune response.
- Chemotaxis and Inflammation: Cytokines like IL-6 and chemokines play a role in recruiting immune cells to the site of inflammation. This continuous recruitment contributes to the chronic inflammatory state observed in autoimmune diseases.
Tissue Damage and Autoimmunity
- Matrix Metalloproteinases (MMPs): Cytokines such as TNF-α and IL-1 stimulate the production of MMPs, enzymes that degrade extracellular matrix components. Excessive MMP activity can lead to tissue destruction, a hallmark of autoimmune diseases.
- Autoantibody Production: Cytokines like IL-6 can promote the differentiation of B cells into antibody-producing plasma cells. Autoantibodies, directed against self-antigens, contribute to tissue damage in autoimmune diseases.
Therapeutic Implications
- Targeting Cytokines: Many current therapies for autoimmune diseases involve the use of biologics that target specific cytokines. For example, anti-TNF therapies aim to reduce the inflammatory effects of TNF-α.
- Immunomodulatory Approaches: Modulating cytokine production or activity is a promising avenue for therapeutic intervention in autoimmune diseases. However, the challenge lies in achieving a balance to prevent immunosuppression.
Role of Cytokines in Organ Transplantation
In organ transplantation, cytokines play a crucial role in modulating immune responses and regulating the complex interplay between donor and recipient cells. Cytokines are signaling molecules that coordinate various cellular activities, and their application in transplantation has shown promising results in managing immunosuppression and mitigating transplant rejection reactions.
One key aspect of cytokine involvement in transplantation is their ability to regulate the immune system. Cytokines such as interleukins (ILs), tumor necrosis factor-alpha (TNF-α), and interferons (IFNs) participate in the communication between immune cells, influencing the balance between pro-inflammatory and anti-inflammatory responses. This modulation is especially critical in preventing the recipient's immune system from recognizing the transplanted organ as foreign and launching an immune attack.
Cytokines contribute significantly to immunosuppression strategies employed in transplantation. For instance, certain interleukins, like IL-2, are targeted to inhibit T cell activation and proliferation, thereby suppressing the immune response that could lead to graft rejection. Additionally, cytokines like transforming growth factor-beta (TGF-β) play a role in inducing immune tolerance by promoting regulatory T cell development, which helps maintain immune homeostasis and prevent excessive inflammatory reactions against the transplant.
Furthermore, understanding and manipulating cytokine networks are essential for managing transplant rejection reactions. Cytokines are intricately involved in the cascade of events leading to rejection, and their targeted modulation can be employed to dampen these responses. By suppressing pro-inflammatory cytokines and promoting anti-inflammatory signals, researchers aim to create an environment conducive to graft acceptance.
Research on cytokines in the context of transplantation is vital for improving transplant success rates and reducing the incidence of rejection. By unraveling the intricate signaling pathways and identifying key cytokines involved, scientists can develop targeted therapies that enhance the effectiveness of immunosuppression while minimizing adverse effects. The ultimate goal is to achieve long-term graft survival with minimal reliance on immunosuppressive drugs, thus improving the overall quality of life for transplant recipients.
Luminex Technology for the Assessment of Cytokines in Immunological Research
Luminex utilizes color-coded microspheres, each coated with a specific capture antibody for a particular cytokine. When these microspheres are exposed to a sample containing cytokines, a fluorescent reporter molecule binds to the captured cytokines, allowing for the quantification of multiple analytes in a single reaction.
Select Service
1. Sample Preparation:
- Begin by collecting biological samples, such as serum, plasma, or cell culture supernatants, where cytokines of interest may be present.
- Ensure proper sample handling and storage to maintain cytokine stability.
2. Assay Setup:
- Select a Luminex kit tailored to the cytokines under investigation.
- Dilute and prepare samples and standards according to kit instructions.
- Mix samples with microspheres coated with specific antibodies for each cytokine.
3. Incubation:
- Incubate the sample-microsphere mixture, allowing cytokines to bind to the corresponding antibodies.
- Wash to remove unbound substances.
4. Detection:
- Introduce a detection antibody labeled with a fluorescent tag that binds to the captured cytokines.
- Utilize a Luminex instrument to measure the fluorescence of each microsphere, quantifying the amount of cytokine present.
5. Data Analysis:
- Employ dedicated software to analyze fluorescence intensity data and generate cytokine concentration values.
- Create standard curves using known cytokine concentrations for accurate quantification.
6. Quality Control:
- Perform internal quality control measures to ensure assay reliability.
- Include appropriate controls and calibrators in each run.
7. Interpretation of Results:
- Evaluate cytokine profiles to understand immune responses or treatment effects.
- Consider the interplay between different cytokines and their concentrations.
Advantages of Luminex Technology
- Multiplexing capability enables the assessment of multiple cytokines in a single assay.
- High sensitivity and wide dynamic range enhance the detection of low-abundance cytokines.
- Requires minimal sample volume compared to traditional methods.
Reference:
- Knop, N., and N. Knop. "Conjunctiva immune surveillance." Ocular Periphery and Disorders (2011): 431.