A Cytokine Release Assay (CRA) is a specialized laboratory technique employed to assess the immune system's response to external stimuli. This assay measures the release of cytokines, which are small, potent proteins central to cell signaling, immune responses, and inflammation. It is grounded in the fundamental understanding that immune cells, when exposed to specific triggers, secrete these cytokines into their surrounding environment.
The Basics of Cytokine Release Assay
At its core, a CRA involves the precise measurement of cytokines released by immune cells in response to specific stimuli. This foundational technique is essential in immunology and biomedical research, shedding light on the behavior of immune cells when exposed to external factors.
Immune Cell Cultivation:
The initial step in a CRA is the isolation and cultivation of immune cells, often peripheral blood mononuclear cells (PBMCs), from a blood sample. PBMCs represent a diverse array of immune cells, making them ideal for analysis.
Stimulation:
Isolated immune cells are then subjected to a precisely controlled stimulus, such as a drug candidate, vaccine component, or immunomodulatory substance. This mimics the in vivo conditions under which the immune system encounters such stimuli.
Incubation:
Following stimulation, immune cells are incubated under controlled laboratory conditions, allowing them to respond to the stimulus. During this incubation period, immune cells release cytokines as part of their response.
Cytokine Measurement:
After incubation, the culture supernatant, containing the released cytokines, is collected. Quantifying these cytokines is a pivotal step in a CRA, providing valuable insights into the immune response's magnitude and characteristics.
Applications of Cytokine Release Assays
Immunogenicity Assessment in Drug Development: CRAs play a vital role in evaluating the immunogenicity of novel therapeutics, particularly biologics. They help gauge the risk of adverse immune reactions to therapeutic agents.
Vaccine Development: CRAs are essential for assessing the immunogenicity of vaccine candidates, providing insights into the immune response elicited by potential vaccines, thus contributing to the development of effective vaccines.
Allergen Testing: CRAs can evaluate the allergenic potential of substances by measuring immune cell responses to allergens, aiding in identifying allergenic triggers, and understanding allergy mechanisms.
Immunotoxicity Assessment: In toxicology studies, CRAs assess the immunotoxic effects of chemicals, environmental pollutants, and pharmaceutical compounds, offering insights into their potential impacts on immune function.
Autoimmune Disease Research: CRAs are applied in autoimmune disease research to investigate immune responses associated with autoimmune conditions, aiding in understanding immune dysregulation mechanisms.
Inflammatory Disease Studies: CRAs are valuable tools for studying inflammatory diseases by assessing immune cell responses to specific stimuli or inflammatory agents, helping unravel the underlying mechanisms of various inflammatory disorders.
Transplantation Research: In transplantation medicine, CRAs evaluate the immune response to graft antigens, contributing to a better understanding of graft rejection mechanisms.
Infectious Disease Studies: Researchers use CRAs to investigate the immune response to infectious agents, facilitating the development of vaccines and treatments for infectious diseases.
Cancer Immunotherapy: In the context of cancer immunotherapy, CRAs assist in evaluating the immunomodulatory effects of therapeutic agents, such as immune checkpoint inhibitors or adoptive cell therapies.
Biomarker Discovery: CRAs identify cytokine release patterns that can serve as potential biomarkers for various conditions or treatment responses.
Environmental and Occupational Health: CRAs assess the immunomodulatory effects of exposure to toxins, pollutants, or hazards in environmental and occupational health studies.
Pharmacological Research: CRAs are valuable tools for pharmacological research, enabling the evaluation of new drug candidates for their immunomodulatory effects.
Different Techniques in Cytokine Release Assays
Enzyme-Linked Immunosorbent Assay (ELISA)
Principle: ELISA is based on the principle of antigen-antibody binding. Specific antibodies are immobilized on a solid phase (typically a microplate), and they capture the target cytokines present in the sample. Detection is achieved using enzyme-conjugated secondary antibodies, and a colorimetric substrate reaction quantifies the cytokine concentration.
Advantages:
- High Specificity and Sensitivity: ELISA offers high specificity for individual cytokines and is sensitive enough to detect low concentrations.
- Quantitative Data: It provides quantitative measurements of cytokine concentrations.
- Versatility: ELISA can be adapted to measure various cytokines and is suitable for research and clinical applications.
Applicable Conditions:
- ELISA is well-suited for cases where specific cytokines need to be measured individually.
- It is commonly used when quantitative data on a single cytokine's concentration is required.
- ELISA is applicable in a wide range of research settings, including immunology, drug development, and clinical diagnostics.
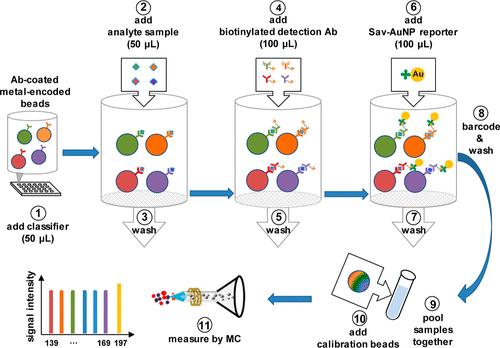 Schematic Diagram for the Multiplexed Bead-Based Sandwich Immunoassays by MC Carried out in a 96-Well Filter Plate (Liu et al., 2022)
Schematic Diagram for the Multiplexed Bead-Based Sandwich Immunoassays by MC Carried out in a 96-Well Filter Plate (Liu et al., 2022)
Multiplex Cytokine Assays with Luminex Technology
Principle: Luminex technology is a multiplex cytokine assay method based on the principles of microspheres and flow cytometry. In Luminex assays, tiny microspheres (each carrying different antibodies) bind to cytokines present in the sample. These microspheres are then injected into a flow cytometer, which can simultaneously detect multiple microspheres tagged with different fluorescent markers, with each marker corresponding to a specific cytokine. By measuring the fluorescence intensity of different microspheres, the concentration of each cytokine can be determined.
Advantages:
- High Multiplexing Capability: Luminex technology allows the simultaneous measurement of multiple cytokines in a single assay, often up to 50 or more, providing a comprehensive cytokine profile.
- Small Sample Size: Luminex assays require relatively small sample volumes, conserving precious samples, especially crucial in clinical or limited-sample scenarios.
- Sensitivity: Luminex-based assays offer high sensitivity, enabling the detection of cytokines at low concentrations, even in complex biological matrices.
- Speed and Efficiency: These assays are known for their speed, with results typically obtained within hours, making them efficient in high-throughput research settings.
Applicable Conditions:
- Luminex technology is ideal when a comprehensive analysis of cytokine profiles is required or when resources and samples are limited.
- It is particularly valuable in high-throughput research environments where rapid analysis of multiple cytokines is essential.
- Luminex technology finds wide application in immunology research, vaccine development, and biomarker discovery.
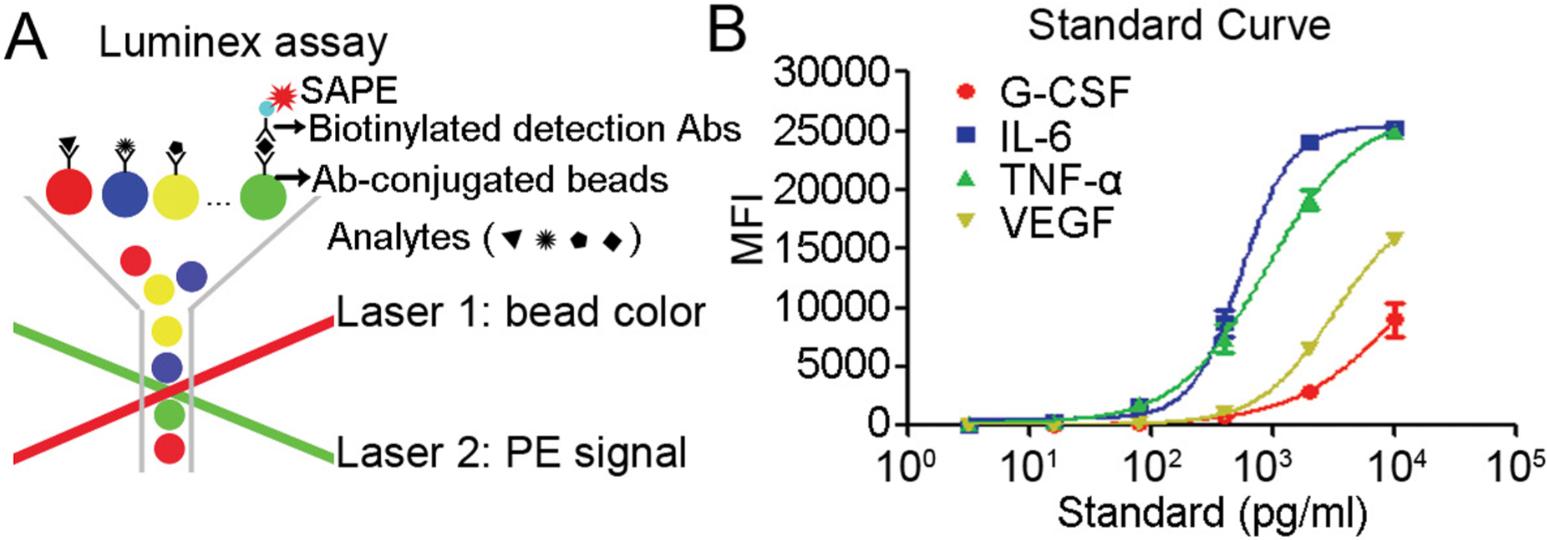 (A) Diagram of Luminex based multiplex assays. (B) Standard curve determination of G-CSF, IL-6, TNF-α, and VEGF (Xie et al., 2014).
(A) Diagram of Luminex based multiplex assays. (B) Standard curve determination of G-CSF, IL-6, TNF-α, and VEGF (Xie et al., 2014).
Flow Cytometry
Principle: Flow cytometry measures cytokine production at the single-cell level. Immune cells are stained with fluorescently labeled antibodies specific to both cytokines and cell surface markers. Cells are then passed through a flow cytometer, and the emitted fluorescence is quantified, allowing the identification of cytokine-producing cells.
Advantages:
- Single-Cell Resolution: Flow cytometry provides detailed insights into cytokine production by individual immune cells.
- Multiparametric Analysis: It can simultaneously measure multiple cytokines and cell surface markers, offering a comprehensive view of immune cell behavior.
- High-Throughput: Flow cytometry can analyze a large number of cells rapidly.
Applicable Conditions:
- Flow cytometry is suitable when studying cytokine production at the single-cell level or investigating complex immune cell interactions.
- It is valuable for immunological studies, cancer immunotherapy, and understanding immune responses in various contexts.
Real-Time Polymerase Chain Reaction (RT-PCR)
Principle: RT-PCR measures cytokine gene expression by quantifying the levels of cytokine mRNA. It involves reverse transcription of mRNA into complementary DNA (cDNA), followed by amplification and quantification using PCR techniques.
Advantages:
- Gene Expression Analysis: RT-PCR provides information on cytokine gene expression, offering insights into gene regulation.
- Quantitative Data: It offers quantitative measurements of cytokine mRNA levels.
- High Specificity: RT-PCR is highly specific for the target genes.
Applicable Conditions:
- RT-PCR is suitable when investigating cytokine gene expression and regulatory mechanisms.
- It is commonly used in molecular biology research and understanding immune responses at the gene expression level.
Bioassays
Principle: Bioassays measure the biological activity of cytokines by assessing their functional effects on target cells or tissues. This may involve cell-based assays, tissue cultures, or functional readouts specific to cytokine activity.
Advantages:
- Functional Assessment: Bioassays directly measure the biological activity of cytokines, providing insights into their functional effects.
- Relevance to In Vivo Responses: They offer a direct link to cytokine activity in biological systems.
- High Specificity: Bioassays can be highly specific for particular cytokine activities.
Applicable Conditions:
- Bioassays are valuable when researchers need to assess the functional impact of cytokines on cells or tissues.
- They are commonly used in drug development, immunotoxicity studies, and investigations of cytokine activities in physiological contexts.
- Each of these techniques offers unique capabilities and can be selected based on the specific research objectives and conditions, providing researchers with a diverse toolkit for cytokine analysis across various scientific disciplines.
References:
- Liu, Jieyi, Bedilu Allo, and Mitchell A. Winnik. "Development of Multiplexed Bead-Based Immunoassays for Profiling Soluble Cytokines and CD163 Using Mass Cytometry." ACS Measurement Science Au 2.6 (2022): 629-640.
- Xie, Songbo, et al. "Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells." PloS one 9.4 (2014): e94496.