Mouse Peripheral Blood Cytokines
At the heart of human health lies a robust immune system, a complex interplay of cellular and molecular components that serves as our body's frontline defense. From the first line of defense, represented by physical barriers like the skin and mucous membranes, to the sophisticated adaptive immune responses driven by T cells and B cells, our immune system is the shield that safeguards us from infections, malignancies, and other threats. Its role extends beyond protection; it participates in tissue repair, homeostasis, and the regulation of bodily functions. A well-functioning immune system is indeed indispensable for our overall well-being.
Central to the immune system's orchestration are cytokines—small, potent signaling proteins secreted by immune cells and various other cell types. These molecular messengers play a pivotal role in mediating communication between immune cells, regulating inflammation, and modulating immune responses. While cytokines are produced in various tissues and organs, their presence in the peripheral blood is of particular significance. The bloodstream acts as a conduit, allowing cytokines to disseminate throughout the body, coordinating immune activities and influencing systemic immune responses. Understanding the dynamics of peripheral blood cytokines is thus paramount in unraveling the intricate workings of the immune system.
In the realm of immunology research, mouse models have emerged as indispensable tools. These small, genetically tractable mammals share remarkable similarities with humans in terms of their immune system. Studying mice allows researchers to dissect the nuances of immune responses, manipulate genes, and investigate the intricate interplay of cytokines in a controlled and ethical manner. The relevance of mouse models extends far beyond basic science; they serve as a bridge between laboratory discoveries and clinical applications. By elucidating the role of peripheral blood cytokines in mice, we gain valuable insights that can be applied to human health, opening doors to innovative therapeutic strategies and novel interventions.
Select Service
Methods for Cytokine Detection in Peripheral Blood
Luminex Multiplex Assay
The Luminex multiplex assay is a commonly used technique to detect multiple cytokines at once in a single sample. It relies on tiny fluorescent beads coated with specific antibodies for different cytokines.
In this method, a blood sample from the mouse is first collected and processed to obtain serum or plasma, which contains the soluble cytokines. These samples are then mixed with the fluorescent beads, each coated with a different antibody designed for a specific cytokine.
When a cytokine in the sample matches its respective antibody-coated bead, a second biotinylated antibody specific to that cytokine is added. This biotinylated antibody attaches to the cytokine, creating a "sandwich" complex.
Next, Streptavidin-phycoerythrin is introduced, which binds to the biotin on the cytokine-antibody complex. The amount of phycoerythrin fluorescence directly corresponds to the cytokine concentration in the sample.
The Luminex system measures the fluorescence signal from each bead, enabling the simultaneous measurement of multiple cytokines in the same sample.
Advantages:
- High-throughput: It can measure multiple cytokines in a single assay, conserving sample volume.
- Sensitivity: It has excellent sensitivity and a wide dynamic range for quantification.
- Multiplexing: It enables the profiling of multiple cytokines simultaneously, providing a comprehensive view of the immune response.
Limitations:
- Cost: The equipment and reagents can be expensive.
- Technical expertise: Skilled personnel are needed to operate and maintain the Luminex system.
- Cross-reactivity: Some cross-reactivity between antibodies may occur in multiplex assays, leading to potential inaccuracies.
Blood Sample Collection and Processing
Blood samples from mice can be gathered through different techniques, including tail vein sampling, retro-orbital bleeding, or cardiac puncture.
After collection, these blood samples are generally allowed to clot, and subsequently, centrifugation is employed to segregate serum or plasma from the cellular elements.
Serum and plasma are the preferred sample types for cytokine analysis since they contain soluble cytokines that have been released into the bloodstream.
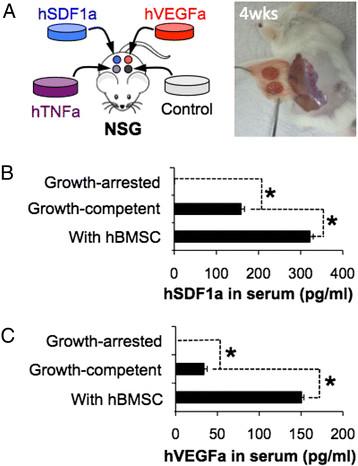 Characterization of human cytokines in peripheral blood of mice carrying different states of genetically engineered stromal cell-laden scaffolds (Lee et al., 2016)
Characterization of human cytokines in peripheral blood of mice carrying different states of genetically engineered stromal cell-laden scaffolds (Lee et al., 2016)
Key Cytokines in Mouse Peripheral Blood
Interleukin-6 (IL-6):
Description: IL-6 is a proinflammatory cytokine produced by various immune cells, such as macrophages and T cells, as well as non-immune cells like fibroblasts.
Functions: IL-6 plays a central role in the acute phase response, promoting inflammation, and stimulating the liver to produce acute phase proteins. It also supports the differentiation of B cells into antibody-producing plasma cells.
Relevance: Elevated IL-6 levels are observed in conditions like rheumatoid arthritis and inflammatory bowel disease, where it contributes to chronic inflammation.
Tumor Necrosis Factor-alpha (TNF-α):
Description: TNF-α is a potent proinflammatory cytokine produced mainly by macrophages but also by other immune cells.
Functions: TNF-α induces inflammation, regulates immune cell activation, and can trigger apoptosis in certain cell types. It plays a crucial role in host defense against infections.
Relevance: TNF-α is a therapeutic target in autoimmune diseases like rheumatoid arthritis and inflammatory bowel disease, where its excessive production contributes to tissue damage.
Interleukin-10 (IL-10):
Description: IL-10 is an anti-inflammatory cytokine produced by various immune cells, including regulatory T cells (Tregs).
Functions: IL-10 dampens immune responses by inhibiting proinflammatory cytokine production and promoting immune tolerance. It helps prevent excessive inflammation and tissue damage.
Relevance: IL-10 is essential for maintaining immune homeostasis, and its deficiency can lead to autoimmunity and exacerbated inflammatory responses.
Interferon-gamma (IFN-γ):
Description: IFN-γ is a proinflammatory cytokine primarily produced by activated T cells and natural killer (NK) cells.
Functions: IFN-γ plays a central role in immune defense against intracellular pathogens, activates macrophages to kill microbes, and enhances antigen presentation by dendritic cells.
Relevance: IFN-γ is associated with autoimmune diseases like multiple sclerosis and contributes to the pathogenesis of conditions such as tuberculosis and chronic viral infections.
Interleukin-4 (IL-4):
Description: IL-4 is an anti-inflammatory cytokine secreted by Th2 cells and other immune cells.
Functions: IL-4 promotes antibody class switching to IgE and IgG, supporting humoral immune responses. It also regulates allergic responses and tissue repair.
Relevance: IL-4 is implicated in allergic diseases like asthma and atopic dermatitis, as well as in autoimmune disorders where immune dysregulation occurs.
Interleukin-2 (IL-2):
Description: IL-2 is a cytokine produced primarily by activated T cells.
Functions: IL-2 is essential for T cell proliferation and activation. It promotes the expansion of effector T cells and the maintenance of regulatory T cells (Tregs).
Relevance: IL-2 is used as a therapeutic agent in cancer immunotherapy and has implications in autoimmune diseases and transplantation.
Mouse Models in Cytokine Research
Significance of Using Mice as Model Organisms in Immunology
Mice have become a cornerstone in immunology research due to their genetic similarity to humans and the availability of advanced genetic manipulation techniques.
Genetic similarity: Many immune-related genes and pathways in mice closely resemble those in humans, making mice an ideal model for studying immune responses and diseases.
Genetic manipulation: The ability to create transgenic and knockout mice allows researchers to investigate the precise roles of specific genes and cytokines in the immune system.
Controlled experiments: Mice offer a controlled and reproducible environment for experimental research, allowing scientists to isolate and study specific aspects of the immune system.
How Mouse Models Help Researchers Understand Cytokine Biology
Manipulating cytokine genes: Researchers can generate mice with specific cytokine genes knocked out or overexpressed, enabling the study of cytokine functions in immune responses and disease.
Disease models: Mouse models are used to mimic human diseases with cytokine involvement, such as inflammatory bowel disease, rheumatoid arthritis, and autoimmune disorders. These models help elucidate the contributions of cytokines to disease pathology.
Therapeutic development: Mouse models are instrumental in preclinical testing of cytokine-based therapies, such as cytokine inhibitors or immune-modulating drugs.
Temporal control: Researchers can induce or block cytokine signaling at specific time points to study their roles at different stages of immune responses.
Specific Mouse Models Contributing to Peripheral Blood Cytokine Knowledge
Cytokine knockout mice: Mouse models with specific cytokine genes knocked out have been instrumental in understanding the functions of individual cytokines. For example, IL-10 knockout mice helped reveal the role of IL-10 in regulating inflammation and immune tolerance.
Transgenic mice: Transgenic mice engineered to overexpress certain cytokines have provided insights into the effects of elevated cytokine levels. For instance, transgenic mice overexpressing TNF-α have been used to study its role in inflammation and autoimmune diseases.
Humanized mice: Mouse models with human immune system components have been developed to study cytokine responses in a more human-like context. These models are crucial for preclinical testing of immunotherapies and vaccine development.
Inducible models: Researchers use inducible mouse models to control the expression of specific cytokines at precise time points. This allows them to investigate the dynamic roles of cytokines in immune responses.
Reference:
- Lee, Jungwoo, Dirk Heckl, and Biju Parekkadan. "Multiple genetically engineered humanized microenvironments in a single mouse." Biomaterials Research 20.1 (2016): 1-13.